Difference between revisions of "How pH affects brewing"
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | {| style="width: | + | {| style="width:860px" |
| − | | | + | |- |
| − | [[Image: | + | [[Image:PH_effects_header.jpg]] |
| − | This is the 2nd in a 3 part series that takes the interested brewer through the topic of pH in brewing. The 1st part ([[An Overview of pH]]) explained the basics that are needed to understand this and the following part. The second part, which is below, examines how pH affects various stages in the brewing process. The 3rd part explains in detail how the mash pH can be affected such that it is at the targeted level. | + | |- |
| + | |||
| + | | This is the 2nd in a 3 part series that takes the interested brewer through the topic of pH in brewing. The 1st part ([[An Overview of pH]]) explained the basics that are needed to understand this and the following part. The second part, which is below, examines how pH affects various stages in the brewing process. The 3rd part explains in detail how the mash pH can be affected such that it is at the targeted level. | ||
As a word of caution, since the following details can be intimidating and cause unjust worries: pH is important during the brewing process, but in most cases the brewer does not have to worry about pH. This is because, if properly done, the brewing processes tend to settle at an adequate pH. As a result pH is one of the last frontiers in brewing practice that home brewers will encounter and worry about. | As a word of caution, since the following details can be intimidating and cause unjust worries: pH is important during the brewing process, but in most cases the brewer does not have to worry about pH. This is because, if properly done, the brewing processes tend to settle at an adequate pH. As a result pH is one of the last frontiers in brewing practice that home brewers will encounter and worry about. | ||
| Line 11: | Line 13: | ||
=enzymatic activity= | =enzymatic activity= | ||
| + | |||
| + | |- | ||
| + | | | ||
[[Image:Enzyme_activity_pH.gif|frame|right|'''Figure 1''' - For each enzyme there is an optimal pH at which it works best. The location and width of that optimum depends on the enzyme. The pH dependent activity change is caused by changes of the enzyme’s structure and electric charges especially at or around the active sites witch hold onto the substrate during the reaction]] | [[Image:Enzyme_activity_pH.gif|frame|right|'''Figure 1''' - For each enzyme there is an optimal pH at which it works best. The location and width of that optimum depends on the enzyme. The pH dependent activity change is caused by changes of the enzyme’s structure and electric charges especially at or around the active sites witch hold onto the substrate during the reaction]] | ||
| Line 21: | Line 26: | ||
The pH sensitivity of enzymes is discussed in further detail in [[Enzymes#The_effect_of_pH Enzymes|The Effect of pH on Enzymes]] | The pH sensitivity of enzymes is discussed in further detail in [[Enzymes#The_effect_of_pH Enzymes|The Effect of pH on Enzymes]] | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
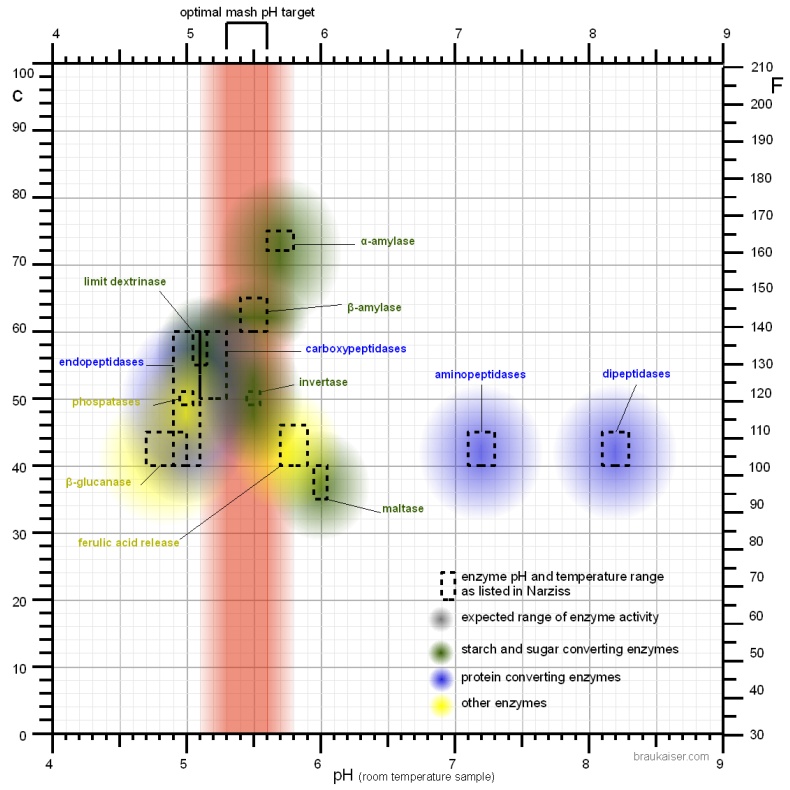
In mashing we tend to target the mash pH to optimize the effectiveness of the most important mash enzymes: the amylases which convert starch into sugar. In room temperature tests the pH optima for alpha amylase has been found at 5.3 and for the beta amylase it is between 5.1 and 5.3 [Briggs, 2004] (see [[Starch_Conversion#pH_and_brewing_water | | In mashing we tend to target the mash pH to optimize the effectiveness of the most important mash enzymes: the amylases which convert starch into sugar. In room temperature tests the pH optima for alpha amylase has been found at 5.3 and for the beta amylase it is between 5.1 and 5.3 [Briggs, 2004] (see [[Starch_Conversion#pH_and_brewing_water | | ||
pH and brewing water in Starch Conversion]]). But when their activity is evaluated at mashing temperatures and the pH of a cooled mash sample is measured it shows their pH optima at 5.7 and 5.4-5.6 respectively. This is the result of a pH shift in the mash that takes place when the mash is heated. In addition to that, the pH optimum of the enzyme is likely to shift as well. As a result, at common starch conversion temperatures (65C/150F) the pH of the mash appears 0.35 units lower than that of a room temperature mash sample [Briggs, 2004]. This needs to be taken into account when looking at pH optima for various mash enzymes. Most commonly, however, the pH optima that are reported in the literature were determined by testing cooled mash samples. | pH and brewing water in Starch Conversion]]). But when their activity is evaluated at mashing temperatures and the pH of a cooled mash sample is measured it shows their pH optima at 5.7 and 5.4-5.6 respectively. This is the result of a pH shift in the mash that takes place when the mash is heated. In addition to that, the pH optimum of the enzyme is likely to shift as well. As a result, at common starch conversion temperatures (65C/150F) the pH of the mash appears 0.35 units lower than that of a room temperature mash sample [Briggs, 2004]. This needs to be taken into account when looking at pH optima for various mash enzymes. Most commonly, however, the pH optima that are reported in the literature were determined by testing cooled mash samples. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
When I used a temperature correcting pH meter to test mash samples at mash temperature and at room temperature, I found a pH shift of only ~ -0.2 pH units. This has been confirmed by other homebrewers and is less than the 0.35 that have been reported by Briggs and other authors. I assume that the apparent 0.35 pH shift is the result of an actual pH shift in the mash and a shift of the pH optimum of the enzyme. | When I used a temperature correcting pH meter to test mash samples at mash temperature and at room temperature, I found a pH shift of only ~ -0.2 pH units. This has been confirmed by other homebrewers and is less than the 0.35 that have been reported by Briggs and other authors. I assume that the apparent 0.35 pH shift is the result of an actual pH shift in the mash and a shift of the pH optimum of the enzyme. | ||
| Line 54: | Line 67: | ||
Even a pH between 5.2-5.4 is already suboptimal for mashes with large amounts of enzymatic weak malts like Munich type malts. Those mashes are better done with a mash pH above 5.4 which will be closer to the pH optimum of the alpha amylase enzyme. In addition to that, when decoction mashing is used the mash pH should not be lower than 5.4 [Kunze, 2007]. While Kunze doesn't give a reason I assume that it is because boiling of the decoctions lowers their pH which may lead to too low of a mash pH for the already enzymatically weakened decoction mash. | Even a pH between 5.2-5.4 is already suboptimal for mashes with large amounts of enzymatic weak malts like Munich type malts. Those mashes are better done with a mash pH above 5.4 which will be closer to the pH optimum of the alpha amylase enzyme. In addition to that, when decoction mashing is used the mash pH should not be lower than 5.4 [Kunze, 2007]. While Kunze doesn't give a reason I assume that it is because boiling of the decoctions lowers their pH which may lead to too low of a mash pH for the already enzymatically weakened decoction mash. | ||
| + | |||
| + | |- | ||
| + | | | ||
==pH optima of enzymes other than the amylases== | ==pH optima of enzymes other than the amylases== | ||
| Line 79: | Line 95: | ||
High mash pH also favors the release of colored malt compounds into the mash [Lewis&Bamforth, 2006]. This is yet another reason why it is beneficial to mash lighter beers at the lower end of the optimal mash pH range. | High mash pH also favors the release of colored malt compounds into the mash [Lewis&Bamforth, 2006]. This is yet another reason why it is beneficial to mash lighter beers at the lower end of the optimal mash pH range. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
| + | |||
| + | |- | ||
| + | | | ||
==conclusion== | ==conclusion== | ||
| Line 88: | Line 111: | ||
* A mash pH between 5.2 and 5.5 is well suited for infusion mashes with enzymatic strong malts | * A mash pH between 5.2 and 5.5 is well suited for infusion mashes with enzymatic strong malts | ||
* A mash pH above 5.4 should be used for decoction mashes and/or enzymatic weak mashes (i.e. large amounts of Munich malt or adjuncts) | * A mash pH above 5.4 should be used for decoction mashes and/or enzymatic weak mashes (i.e. large amounts of Munich malt or adjuncts) | ||
| + | |||
| + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| + | |- | ||
| + | | | ||
=Extraction of Tannins= | =Extraction of Tannins= | ||
| Line 94: | Line 121: | ||
But why do some beers have a problem with tannins while others don't? This too has to do with pH. The solubility of tannins in water is affected by 2 factors. One of them is temperature and the other one is pH. The effect of temperature is obvious. The higher the temperature the more soluble most compounds including tannins generally are and the higher the rate at which they are leached into the wort. | But why do some beers have a problem with tannins while others don't? This too has to do with pH. The solubility of tannins in water is affected by 2 factors. One of them is temperature and the other one is pH. The effect of temperature is obvious. The higher the temperature the more soluble most compounds including tannins generally are and the higher the rate at which they are leached into the wort. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
But the effect of pH is more profound. Polyphenols, which tannins are, are weak acids. And as we saw in [[An Overview of pH#weak_acids_and_bases|acids and bases]] they readily donate protons to their environment if the concentration of H<sup>+</sup> is low enough, i.e. the pH is high enough. Once a polyphenol molecule looses one or more protons it becomes negatively charged. Due to the water molecule's charged nature, one side is positive and the other is negative, water is a great solvent for charged molecules and that's why the solubility of tannins increases when pH rises [Bogspot.com, 2009]. | But the effect of pH is more profound. Polyphenols, which tannins are, are weak acids. And as we saw in [[An Overview of pH#weak_acids_and_bases|acids and bases]] they readily donate protons to their environment if the concentration of H<sup>+</sup> is low enough, i.e. the pH is high enough. Once a polyphenol molecule looses one or more protons it becomes negatively charged. Due to the water molecule's charged nature, one side is positive and the other is negative, water is a great solvent for charged molecules and that's why the solubility of tannins increases when pH rises [Bogspot.com, 2009]. | ||
A generally accepted pH threshold is 5.8-6.0 [source?]. As a result, the mash pH is generally low enough to prevent excessive tannin extraction. But sparging with high alkalinity water can quickly consume the mash's buffer capacity and lead to pH levels that lead to excessive tannin extraction into the wort. This is because the high concentration of carbonates and bicarbonates in the water forms a strong buffer. As the sweet wort is diluted and with it the mash’s ability to buffer its pH at a level closer to the mash pH, the sparge water and its pH are taking over which can raise the pH above 6.0 and cause excessive tannin extraction. | A generally accepted pH threshold is 5.8-6.0 [source?]. As a result, the mash pH is generally low enough to prevent excessive tannin extraction. But sparging with high alkalinity water can quickly consume the mash's buffer capacity and lead to pH levels that lead to excessive tannin extraction into the wort. This is because the high concentration of carbonates and bicarbonates in the water forms a strong buffer. As the sweet wort is diluted and with it the mash’s ability to buffer its pH at a level closer to the mash pH, the sparge water and its pH are taking over which can raise the pH above 6.0 and cause excessive tannin extraction. | ||
| + | |||
| + | |- | ||
| + | | | ||
There are a number of ways that this pH raise during sparging can be prevented or at least mitigated such that the pH is not allowed to raise above 5.8: | There are a number of ways that this pH raise during sparging can be prevented or at least mitigated such that the pH is not allowed to raise above 5.8: | ||
| − | * '''Limited sparging:''' If sparging is stopped before the pH of the water, that most of the grain sits in, rises above 6.0 an excessive amount of tannins will not be extracted into the boil kettle. While this limits the efficiency of the lauter, it is the most practical way of controlling tannin extraction from the grain husks. It also complies with the Reinheitsgebot (German beer purity law). An elegant way of limiting the amount of sparging is lowering the amount of water used for | + | * '''Limited sparging:''' If sparging is stopped before the pH of the water, that most of the grain sits in, rises above 6.0 an excessive amount of tannins will not be extracted into the boil kettle. While this limits the efficiency of the lauter, it is the most practical way of controlling tannin extraction from the grain husks. It also complies with the Reinheitsgebot (German beer purity law). An elegant way of limiting the amount of sparging is lowering the amount of water used for sparging and increasing the amount of water used for mashing. Pilsner beers, which are delicate beers that would suffer from excessive tannin extraction, are brewed with a mash thickness of up to 5.5 l/kg (2.5 qt/lb) which limits the amount of water that is available for sparging [Narziss, 2005] |
| − | * '''Low alkalinity sparge water:''' The buffer capacity (alkalinity) of soft water is not strong enough to counteract the buffer of the mash even at high dilution rates. If brewing water is build from distilled or reverse osmosis water the salt additions destined for the sparge water can be made in the kettle while | + | * '''Low alkalinity sparge water:''' The buffer capacity (alkalinity) of soft water is not strong enough to counteract the buffer of the mash even at high dilution rates. If brewing water is build from distilled or reverse osmosis water the salt additions destined for the sparge water can be made in the kettle while “plain” water is used for sparging. For brewers who care, this method is not approved by the Reinheitsgebot. |
| − | * '''Sparge water acidification:''' The sparge water alkalinity can be reduced though acid additions and its pH can be lowered to a pH of 6 where it will | + | * '''Sparge water acidification:''' The sparge water alkalinity can be reduced though acid additions and its pH can be lowered to a pH of 6 where it will only have a weak buffer capacity and will not be able to significantly counteract the pH that is set by the mash’s strong buffer. It is compliant with the Reinheitsgebot if lactic acid is used that was derived from malt based fermentation with malt derived lactobacillus. |
| + | |||
| + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| + | |- | ||
| + | | | ||
=Protein coagulation= | =Protein coagulation= | ||
| − | [[Image:Batch_77_bruchbilder.jpg|frame|right|'''Figure 3''' - Wort clarity and break formation before, at the beginning, and at the end of the boil. The starting boil pH was 5.35 and the cast out wort pH was 5.30. It can be seen that the wort 10 min before boiling is still clear. | + | [[Image:Batch_77_bruchbilder.jpg|frame|right|'''Figure 3''' - Wort clarity and break formation before, at the beginning, during, and at the end of the boil. The starting boil pH was 5.35 and the cast out wort pH was 5.30. It can be seen that the wort 10 min before boiling is still clear. At the beginning of the boil protein have coagulated to form a haze. Shortly after that, they started to form flocks (hot break) which cleared up the wort. At the end of the 60 min boil the flocks are larger while the wort is brilliantly clear]] |
As we saw with the enzyme activity and the tannin extraction the main effect that a changing pH has is its ability to change the electrical charges on molecules. And that is no different with proteins during wort boiling. | As we saw with the enzyme activity and the tannin extraction the main effect that a changing pH has is its ability to change the electrical charges on molecules. And that is no different with proteins during wort boiling. | ||
| − | But in addition to that, another structural aspect of proteins matters. Proteins are chains of amino acids that are folded into a structure that allows them to do their job in an organism. Some of these amino acids react well with water, they are called hydrophilic, while others try to avoid water | + | But in addition to that, another structural aspect of proteins matters. Proteins are chains of amino acids that are folded into a structure that allows them to do their job in an organism. Some of these amino acids react well with water, they are called hydrophilic, while others try to avoid water and they are called hydrophobic. In water soluble proteins the hydrophobic amino acids are oriented such that they face towards the center of the protein and therefore can react with each other rather than with the surrounding water while the hydrophilic ones face outward. |
| − | When the protein coagulates | + | When the protein coagulates its chain of amino acids is not broken but it looses its folded structure and some of the hydrophobic amino acids will face outward. Because hydrophobic amino acids rather react with other hydrophobic amino acids they try to avoid water by joining with hydrophobic amino acids of other coagulated proteins. As a result the proteins clump together and form the flocks we all know as hot and cold break. In addition to that tannins from the husks and hop material, which are present in wort, will attach themselves to these hydrophobic areas. Through hydrogen bonds they will form bridges between coagulated proteins. The aforementioned hydrophobic protein areas are generally rich in the amino acid proline, |
| − | + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | |
| + | |- | ||
| + | | | ||
| − | + | But how does pH affect this? This is where the concept of the isoelectric point, which we discussed in [[An Overview of pH#isoelectric_point_.28IEP.29|isoelectric point]], plays a role. When the overall charge on the proteins is neutral, which it is at their isoelectric point, they don't repel each other anymore. Such repulsion would be the case at a pH lower or higher than the iso-electric point where the proteins are predominantly negatively or positively charged respectively. When they don't repel each other, hydrophobic sections of the denatured proteins can react with each other more easily and they'll clump together faster. The isoelectric point is also where the solubility of a protein is at its lowest level since uncharged molecules don't react with water as well as charged ones. | |
| − | During boiling the pH drops by about 0.1 – 0.2 pH units from 5. | + | All this helps protein coagulation during the boil. The iso-electric point of wort proteins is around 4.9 [BrewingTechniques, 1993]. But a common boil pH of 5.2 – 5.4 (room temperature sample) does not reach a pH that low and therefore the protein coagulation is not as good as it could be [Narziss, 2005]. While it is possible to lower the boil pH through the addition of acids, it is generally not done because the level of protein coagulation that is achieved at a pH of 5.2 is sufficient and reduction of the boil pH further reduces the hop alpha acid utilization. |
| + | |||
| + | During boiling the pH drops by about 0.1 – 0.2 pH units from 5.3 – 5.5 pH to about 5.2 – 5.3 pH. This may be due to the addition of bitter acids from the hops, formation of acidic Maillard products, precipitation of alkaline phosphates or the reaction of polypeptides with calcium, liberating protons [Briggs, 2004]. | ||
| + | |||
| + | |- | ||
| + | | | ||
=Hop utilization= | =Hop utilization= | ||
| Line 125: | Line 170: | ||
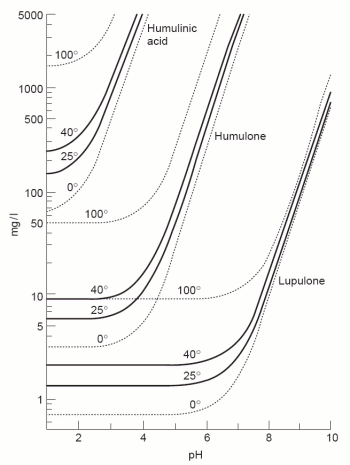
[[Image:Alpha_acid_solubility.gif|frame|right| '''Figure 4''' – The solubility of various hop alpha acids based on temperature and pH. This chart was taken from [Briggs, 2004]]] | [[Image:Alpha_acid_solubility.gif|frame|right| '''Figure 4''' – The solubility of various hop alpha acids based on temperature and pH. This chart was taken from [Briggs, 2004]]] | ||
| − | Before hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved into the boiling wort. That solubility depends on both wort temperature and pH. Except for elevation and equipment dependent variations the boil temperature is largely constant. But boil pH can easily vary and with it the solubility of hop acids. It has been shown that this solubility increases with pH (see Figure 4) [Briggs, 2004] which is why the bitterness extraction from hops is greater at a higher boil pH. Many brewers, however, have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher compared to bitterness gained from a boil at a lower pH. | + | Before hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved into the boiling wort. That solubility depends on both wort temperature and pH. Except for elevation and equipment dependent variations, the boil temperature is largely constant. But boil pH can easily vary and with it the solubility of hop acids. It has been shown that this solubility increases with pH (see Figure 4) [Briggs, 2004] which is why the bitterness extraction from hops is greater at a higher boil pH. Many brewers, however, have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher compared to bitterness gained from a boil at a lower pH. |
The same mechanism that increases tannin solubility at higher pH increases the solubility of hop resins. Hop resins behave acidic and want to loose H<sup>+</sup> at higher pH. That leaves a charged molecule which in turn is more soluble in water. | The same mechanism that increases tannin solubility at higher pH increases the solubility of hop resins. Hop resins behave acidic and want to loose H<sup>+</sup> at higher pH. That leaves a charged molecule which in turn is more soluble in water. | ||
| − | The reduction in solubility of hop acids can be seen during fermentation. After | + | The reduction in solubility of hop acids can be seen during fermentation. After wort is pitched with yeast and the pH falls during fermentation, most of the unisomerized hop resins will come out of solution again. They become part of the brown gunk that floats on top of the Kraeusen which, if a smooth bitterness is desired, should be removed via blow-off tube, skimming and not allowed to fall back into the beer [Narziss, 2005][Kunze, 2007]. |
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
=Maillard reactions= | =Maillard reactions= | ||
| − | [[Image:Experiment_normal_vs_high_pH.jpg|frame|right|'''Figure 5''' - Wort boiled for 15 min at pH 5.5 (left) and pH 6.5 (right). The increased color of the higher pH wort is remarkable. It is a result of | + | [[Image:Experiment_normal_vs_high_pH.jpg|frame|right|'''Figure 5''' - Wort boiled for 15 min at pH 5.5 (left) and pH 6.5 (right). The increased color of the higher pH wort is remarkable. It is a result of stronger Maillard reactions at higher pH.]] |
Maillard reactions, or non-enzymatic browning, start with a reaction between an amino acid and a reducing sugar. The final products are melanoidins which are responsible for the darkening of both, malt during kilning and wort during boiling. | Maillard reactions, or non-enzymatic browning, start with a reaction between an amino acid and a reducing sugar. The final products are melanoidins which are responsible for the darkening of both, malt during kilning and wort during boiling. | ||
| − | The rate of Maillard reactions is affected by wort concentration, temperature and pH. Here we want to have a look at the effects of pH during the wort boil. The higher pH the faster the rate of Maillard reactions will be. I was able to observe that during my mashing experiments when I boiled the wort from a 5.5 pH and a 6.5 pH mash. The result is shown in Figure 5. While the difference is dramatic despite the short boil time of only 15 min, a wort boil pH of 6.5 is well out of the normal range. A beer brewed with that of a boil pH will have more severe problems than just increased color. But in general, aiming for a lower boil pH (5.3 - 5.4 for a room temperature sample taken at the beginning of the boil) will keep lighter beers from picking up too much color during boiling. | + | The rate of Maillard reactions is affected by wort concentration, temperature and pH. Here we want to have a look at the effects of pH during the wort boil. The higher pH the faster the rate of Maillard reactions will be. I was able to observe that during my mashing experiments when I boiled the wort from a 5.5 pH and a 6.5 pH mash. The result is shown in Figure 5. While the difference is dramatic, despite the short boil time of only 15 min, a wort boil pH of 6.5 is well out of the normal range. A beer brewed with that of a boil pH will have more severe problems than just increased color. But in general, aiming for a lower boil pH (5.3 - 5.4 for a room temperature sample taken at the beginning of the boil) will keep lighter beers from picking up too much color during boiling. |
But how is pH affecting the rate of Maillard reactions? It is through its affect on the amino acid. As mentioned earlier, Maillard reactions start with the reaction between a reducing sugar and an amino acid. That reaction yields so called Amadori products which are an important intermediate during the Maillard reaction. This initial reaction happens at a much higher rate when the amino group of the amino acid becomes more likely to donate its electrons (Nucleophilic) to the bond with the reducing sugar. That is the case when the amino group lost a proton (H<sup>+</sup>) or is on the edge of loosing it to counteract the low H<sup>+</sup> concentration (i.e. high pH) of its environment. [khymos.org] | But how is pH affecting the rate of Maillard reactions? It is through its affect on the amino acid. As mentioned earlier, Maillard reactions start with the reaction between a reducing sugar and an amino acid. That reaction yields so called Amadori products which are an important intermediate during the Maillard reaction. This initial reaction happens at a much higher rate when the amino group of the amino acid becomes more likely to donate its electrons (Nucleophilic) to the bond with the reducing sugar. That is the case when the amino group lost a proton (H<sup>+</sup>) or is on the edge of loosing it to counteract the low H<sup>+</sup> concentration (i.e. high pH) of its environment. [khymos.org] | ||
In short: high pH -> more amino acids have lost a proton from their amino groups -> they are now more likely to donate an electron to the reducing sugar -> more Amadori products -> more melanoidens. | In short: high pH -> more amino acids have lost a proton from their amino groups -> they are now more likely to donate an electron to the reducing sugar -> more Amadori products -> more melanoidens. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
=Nutrient uptake by yeast= | =Nutrient uptake by yeast= | ||
| Line 149: | Line 202: | ||
Maltose uptake is a proton symport process through the cell membrane. The proton concentration outside the cell is greater (lower pH) than inside the cell (higher pH) and therefore a natural gradient exists which encourages protons to flow from the outside to the inside of the cell. Though the use of a symporter, a cell membrane protein, maltose can “piggy back” on the flow of protons into the cell. This is one of the reasons why yeast cells do better in an acidic environment and have means of lowering the pH. | Maltose uptake is a proton symport process through the cell membrane. The proton concentration outside the cell is greater (lower pH) than inside the cell (higher pH) and therefore a natural gradient exists which encourages protons to flow from the outside to the inside of the cell. Though the use of a symporter, a cell membrane protein, maltose can “piggy back” on the flow of protons into the cell. This is one of the reasons why yeast cells do better in an acidic environment and have means of lowering the pH. | ||
| − | The ability of yeast to lower the pH | + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] |
| + | |- | ||
| + | | | ||
| + | |||
| + | The ability of yeast to lower the beer’s pH is important for healthy and low yeast stress fermentation and is one of the reasons why sufficient pitching rates are important and why it is better to step up starters rather than starting a small amount of yeast in a large starter. The more yeast cells that are working on lowering the pH the faster the pH will be able to drop. | ||
| + | |||
| + | As yeasts age, starve or otherwise loose their vitality, it becomes increasingly difficult for them to pump H<sup>+</sup> from their cells into the beer. After all, this goes against nature’s desire to equalize everything and therefore takes energy. The result is a slight rise of the beer pH after primary fermentation. The pH can rise more significantly if the beer is not taken off the yeast before a large number of yeast cells start to autolyze. | ||
| − | + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | |
| + | |- | ||
| + | | | ||
=Inhibition of beer spoilage organisms= | =Inhibition of beer spoilage organisms= | ||
| − | Besides alcohol and hop resins, the low pH of beer, which is generally lower than 4.5, is one of the main reasons why it provides a poor growth medium for many bacteria. There are for example no pathogens (bugs that can make you sick like E. coli or C. botulinum) that can grow in beer and it is therefore safe to | + | Besides alcohol and hop resins, the low pH of beer, which is generally lower than 4.5, is one of the main reasons why it provides a poor growth medium for many bacteria. There are for example no pathogens (bugs that can make you sick like E. coli or C. botulinum) that can grow in beer and it is therefore safe to even drink beers that have been infected. |
Wort tends to have a pH between 5.2 and 5.6 which allows the growth of pathogens and care should be taken if it is stored unpitched and unrefrigerated for an extended period of time. The FDA requires that all canned food stuff with a pH greater than 4.6 needs to be heat treated under pressure to allow for temperatures that are high enough for sterilization in order to eliminate pathogens and their spores. That's why wort needs to be pressure canned or frozen to be stored. | Wort tends to have a pH between 5.2 and 5.6 which allows the growth of pathogens and care should be taken if it is stored unpitched and unrefrigerated for an extended period of time. The FDA requires that all canned food stuff with a pH greater than 4.6 needs to be heat treated under pressure to allow for temperatures that are high enough for sterilization in order to eliminate pathogens and their spores. That's why wort needs to be pressure canned or frozen to be stored. | ||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
=Clarifies in kettle and fermenter= | =Clarifies in kettle and fermenter= | ||
| + | |||
| + | [[Image:Fining_with_gelatin.jpg|frame|right|'''Figure 6''' – How gelatin settles yeast. At beer pH gelatin’s large molecules carry many positive charges. These charges attract the negatively charged yeast cells. The size of the gelatin molecules cause them to settle while taking the yeast cells with them.]] | ||
Brewers may use fining agents in the kettle and in the finished beer. Depending on the product used it aids the precipitation of proteins, yeast cells or tannins but all aim at reducing beer haze and through it improve clarity and haze stability. Here we want to focus on clarifiers that work through electrostatic forces. This is the case for collagen's (Gelatin or Isinglass) affect on yeast cells and to some extend the case for carrageen's (Irish Moss or Whirlfloc) affect on wort proteins. Other clarifiers like silica gel and tannic acid bind with proteins through reactions with their hydrophobic regions. | Brewers may use fining agents in the kettle and in the finished beer. Depending on the product used it aids the precipitation of proteins, yeast cells or tannins but all aim at reducing beer haze and through it improve clarity and haze stability. Here we want to focus on clarifiers that work through electrostatic forces. This is the case for collagen's (Gelatin or Isinglass) affect on yeast cells and to some extend the case for carrageen's (Irish Moss or Whirlfloc) affect on wort proteins. Other clarifiers like silica gel and tannic acid bind with proteins through reactions with their hydrophobic regions. | ||
| − | Collagen is a very effective clarifier which is used after fermentation is complete. It is the effective compound in isinglass and gelatin. Collagen is a protein that, depending on its origin and processing has an isoelectic point of ~5.5 (isinglass) [Alton], 7.0 – 9.0 (type A gelatin) or 4.7-5.4 (type B gelatin) [GMIA]. Isinglass is produced from the swim bladder of tropical fish | + | Collagen is a very effective clarifier which is used after fermentation is complete. It is the effective compound in isinglass and gelatin. Collagen is a protein that, depending on its origin and processing has an isoelectic point of ~5.5 (isinglass) [Alton], 7.0 – 9.0 (type A gelatin) or 4.7-5.4 (type B gelatin) [GMIA]. Isinglass is produced from the swim bladder of tropical fish; gelatin on the other hand is either made from pig skin and feet (type A) or bovine (cow) parts (type B). |
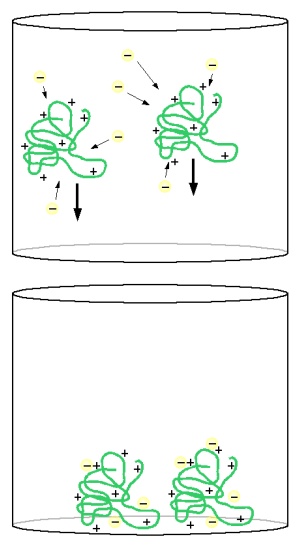
| − | [[ | + | For all of these sources of collagen the IEP (isoelectric point) is above the pH of beer which means that the collagen molecules carry a net positive charge. That was shown in [[An Overview of pH#Isoelectric_point_.28IEP.29|Isoelectric point]]. Yeas cells, however, are negatively charged. After all, they actively pump protons, which carry a positive charge, into their environment. The different polarity of their electrical charges cause the yeast to be attracted to the collagen molecules. Due to its molecule size the collagen slowly settles to the bottom of the vessel and takes the yeast with it. |
| − | + | Gelatin and isinglass are not only known to aid yeast settlement but remove protein hazes as well. Proteins in beer are largely positively charged (the same charge as the collagen) and gelatin and isinglass will show little to no effect on the protein part of beer hazes. However, protein haze is formed by bonds between tannins and protein and it is the tannins that collagen bonds with. When it then settles to the bottom it takes the protein-tannin complexes, which cause the haze, with it. | |
| − | + | Because of its wide availability, almost every grocery store carries it, most brewers use Knox unflavored gelatin if they need to clarify a beer. Knox gelatin is made from pork and therefore type A [Kraftfoods]. Whenever I use it I use about 3-4 g (½ packets) dissolved in 100-200 ml warm water for a 16-20 l (4-5 gal) batch of beer. The water should be sanitary and sanitary procedures need to be followed since the gelatin suspension cannot be boiled. Once the gelatin is completely dissolved, the mix is added to the beer and mixed in well. It should take only a few days for the gelatin to settle after which the clear beer can be drawn off. | |
| − | + | ||
| − | Because of its wide availability, almost every grocery store carries it, most brewers use Knox unflavored gelatin if they need to clarify a beer. Knox gelatin is made from pork and therefore type A [Kraftfoods]. Whenever I use it | + | |
Clarifiers can also be used in the boil kettle. Popular products are Irish Moss and Whirlfloc. The active ingredient in both is carrageen. In contrast to collagen carrageen is a polysaccharide that is strongly negatively charged over the pH range encountered in brewing [Martin, 2000]. But at boil pH most proteins are at or slightly above their isoelectric point which means they carry a neutral or slightly negative charge and should therefore not be attracted to carrageen. In fact the use of carrageen (e.g. Irish Moss) in the boil does not increase the protein precipitation but it causes the formation of larger flocks that settle more quickly and form better trub cones in the whirlpool [Lewis&Bamforth, 2006]. | Clarifiers can also be used in the boil kettle. Popular products are Irish Moss and Whirlfloc. The active ingredient in both is carrageen. In contrast to collagen carrageen is a polysaccharide that is strongly negatively charged over the pH range encountered in brewing [Martin, 2000]. But at boil pH most proteins are at or slightly above their isoelectric point which means they carry a neutral or slightly negative charge and should therefore not be attracted to carrageen. In fact the use of carrageen (e.g. Irish Moss) in the boil does not increase the protein precipitation but it causes the formation of larger flocks that settle more quickly and form better trub cones in the whirlpool [Lewis&Bamforth, 2006]. | ||
| Line 177: | Line 241: | ||
The use of Irish Moss or Whirlfloc is not permitted by the German purity law. Neither is gelatin, but isinglass can be used if the beer is subsequently filtered [Narziss, 2005]. | The use of Irish Moss or Whirlfloc is not permitted by the German purity law. Neither is gelatin, but isinglass can be used if the beer is subsequently filtered [Narziss, 2005]. | ||
| + | |- | ||
| + | | | ||
=Beer taste= | =Beer taste= | ||
| Line 182: | Line 248: | ||
How pH changes the taste becomes evident if the taste of a post boil wort sample and that of very young beer is compared. At the beginning of fermentation yeast lowers the pH while no or only little fermentation has happened yet. As a result the sample with yeast will taste sweeter and fresher than the post boil sample which tastes more dull even though they both have a nearly identical sugar profile. | How pH changes the taste becomes evident if the taste of a post boil wort sample and that of very young beer is compared. At the beginning of fermentation yeast lowers the pH while no or only little fermentation has happened yet. As a result the sample with yeast will taste sweeter and fresher than the post boil sample which tastes more dull even though they both have a nearly identical sugar profile. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
=References= | =References= | ||
| Line 199: | Line 269: | ||
| − | ''This text attempts to be a comprehensive list of the effects that pH has on brewing processes. If you know of other effects pH has | + | ''This text attempts to be a comprehensive list of the effects that pH has on brewing processes. If you know of other effects pH has contact me (kai at braukaiser dot com) and I may find the time to look into it and include it in this list.'' |
|} | |} | ||
Latest revision as of 15:33, 25 September 2009
| This is the 2nd in a 3 part series that takes the interested brewer through the topic of pH in brewing. The 1st part (An Overview of pH) explained the basics that are needed to understand this and the following part. The second part, which is below, examines how pH affects various stages in the brewing process. The 3rd part explains in detail how the mash pH can be affected such that it is at the targeted level.
As a word of caution, since the following details can be intimidating and cause unjust worries: pH is important during the brewing process, but in most cases the brewer does not have to worry about pH. This is because, if properly done, the brewing processes tend to settle at an adequate pH. As a result pH is one of the last frontiers in brewing practice that home brewers will encounter and worry about. In brewing pH affects: Contentsenzymatic activity | |
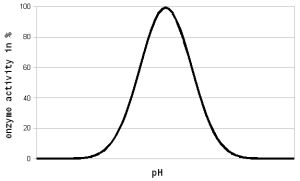 Figure 1 - For each enzyme there is an optimal pH at which it works best. The location and width of that optimum depends on the enzyme. The pH dependent activity change is caused by changes of the enzyme’s structure and electric charges especially at or around the active sites witch hold onto the substrate during the reaction All enzymes are proteins which rely on their shape for their function. This shape is primarily determined by the sequence of amino acids, which are the building blocks of proteins, and secondarily by interactions between these amino acids. The sequence of amino acids determines how the protein is folded into a shape that allows it to perform its function. That shape is also affected by electrostatic forces between electrically changed amino acids in the protein. These electrostatic forces cause charged amino acids to repel (same charge) or attract (opposite charge) each other. As we saw in the first part, the distribution of electrical charges on a molecule depends on the pH of the environment. A high pH (low H+ concentration) will cause more amino acids to donate protons into the solution which leaves the remaining amino acid negatively changed, whereas a low pH (high H+ concentration) has the opposite effect. Other amino acids will accept H+ from the solution and therefore change to having a positive charge as the pH falls. In order for enzymes to function a proper balance of these charged amino acids needs to be present. That balance will exist at and around the pH at which the enzyme is most effective. As long as the difference between the optimal and actual pH is low (1-2 pH units) the effect of pH on the enzyme activity is reversible since the enzyme is not permanently damaged (denatured). In practice this means that pH can be corrected even after dough-in and little or no enzymes will be lost as a result of a suboptimal initial pH. But once the pH is adjusted to an adequate level these enzymes will get a later start. At that point some of them may have already been denatured as a result of the heat before they got a chance to work at an optimal pH. This is especially true for the more heat liable beta-amylase and limit dextrinase. If the pH is substantially far from the optimum the enzyme can also be denatured, but that is difficult to achieve in brewing. The pH sensitivity of enzymes is discussed in further detail in The Effect of pH on Enzymes |
|
|
In mashing we tend to target the mash pH to optimize the effectiveness of the most important mash enzymes: the amylases which convert starch into sugar. In room temperature tests the pH optima for alpha amylase has been found at 5.3 and for the beta amylase it is between 5.1 and 5.3 [Briggs, 2004] (see pH and brewing water in Starch Conversion). But when their activity is evaluated at mashing temperatures and the pH of a cooled mash sample is measured it shows their pH optima at 5.7 and 5.4-5.6 respectively. This is the result of a pH shift in the mash that takes place when the mash is heated. In addition to that, the pH optimum of the enzyme is likely to shift as well. As a result, at common starch conversion temperatures (65C/150F) the pH of the mash appears 0.35 units lower than that of a room temperature mash sample [Briggs, 2004]. This needs to be taken into account when looking at pH optima for various mash enzymes. Most commonly, however, the pH optima that are reported in the literature were determined by testing cooled mash samples. |
|
|
When I used a temperature correcting pH meter to test mash samples at mash temperature and at room temperature, I found a pH shift of only ~ -0.2 pH units. This has been confirmed by other homebrewers and is less than the 0.35 that have been reported by Briggs and other authors. I assume that the apparent 0.35 pH shift is the result of an actual pH shift in the mash and a shift of the pH optimum of the enzyme. Most homebrewers measure the pH of a cooled mash sample when checking pH. While pH meters are able to measure the hot mash pH it is not advisable to do so because it shortens the life of the probe. If colorpHast strips are used in the hot mash, their color reaction is the same or very close to their color reaction in a room temp mash sample. This means that these strips produce the same pH reading regardless of temperature (at least when used in mash or wort). But it should be kept in mind that these strips report a pH that is about 0.3 pH lower than the actual pH (see colorpHast strips in brewing). The pH targets, optima and measurements listed in this article have been determined with room temperature samples unless otherwise noted. A commonly accepted optimal range for mash pH is 5.2 - 5.7 with 5.5 being optimal for starch conversion activity but many authors report wort and beer quality benefits if the pH is lowered into the 5.2 - 5.4 range [Kunze, 2007][Narziss, 2005]. Kunze in particular lists the following benefits for a mash pH as low as 5.2. Since it is a good and fairly comprehensive list I cited it here. Some of these benefits listed will be explained in the following sections [Kunze, 2007]:
The pH of the mash should not fall below 5.2. At this point the activity of the amylase enzymes starts to suffer significantly (see mash pH experiments). This is particularly important if sour mashing is used to create sour beers and not just as a means for adjusting mash pH. Just enough sour mash should be added to correct the pH and the rest is added towards the end of the mash when the conversion of the main mash is complete. Even a pH between 5.2-5.4 is already suboptimal for mashes with large amounts of enzymatic weak malts like Munich type malts. Those mashes are better done with a mash pH above 5.4 which will be closer to the pH optimum of the alpha amylase enzyme. In addition to that, when decoction mashing is used the mash pH should not be lower than 5.4 [Kunze, 2007]. While Kunze doesn't give a reason I assume that it is because boiling of the decoctions lowers their pH which may lead to too low of a mash pH for the already enzymatically weakened decoction mash. | |
pH optima of enzymes other than the amylasesThe following is a collection of the pH optimum data that is given in Narziss' “Abriss der Bierbrauerei” [Narziss, 2005]. While the targeted mash pH is generally not determined by enzymes other than the amylase enzymes it also has an effect on other enzymes which may be considered in certain mashing schedules. If a ferulic acid rest is held at 35-40C (95-105F) the release of ferulic acid is reduced if the pH is below 5.7 [Narziss, 2005]. Because of that it is advisable to add any acidulated malt, which may be part of the grist for pH correction, after that rest has been completed. Very lightly kilned malts contain the enzyme phytase which is able to release phosphate from phytin present in the malt [deLange, 2004]. That release of phosphate serves to lower the mash pH during the so called acid rest. It not only lowers the mash pH, it also increases the buffer capacity of the mash. This increased buffer capacity may make it later more difficult for the yeast to lower the beer pH [Kolbach, 1953]. The enzyme has a pH optimum of about 5.0 which is the reason for increased buffer capacity of worts produced from lightly kilned malts (e.g. Pilsner malt) with lower pH mashes. The β-glucanase, which is most active between 40 and 50 C (104 – 122 F) and degrades beta β-glucanes, likes it a little more acidic. It’s pH optimum is between 4.7 and 5.0. In malt 4 groups of protein degrading enzymes (proteases) exist. Endopeptidases are the protein equivalent of α-amylase; they break peptide bonds of proteins and peptides within the molecule. Carboxypeptdiases are like β-amylase; they break off amino acids from the carbonyl end of the protein molecules. Dipeptidases are like the enzyme maltase, which breaks maltose in two glucose molecules, only that they break a dipeptide into two amino acid molecules. And the last group are the aminopeptidases which act like carboxypeptidases but attack the protein from its amino end. Narziss lists the following pH optima for these groups:
Only endopeptidases and carboxypeptidases are active at mash pH levels. The mash environment is too acidic for effective dipeptidase and aminopeptiodase activity. Compared to the amylases these enzymes prefer a slightly more acidic environment which explains the higher release of amino acids in lower pH mashes. If necessary, pH can also be used to limit these protein degrading enzymes by lowering the mash pH not until the saccharification temperature range 60-70 C (140-160 F) is reached. This is only of interest if dough-in below the saccharification temperature range was necessary. High mash pH also favors the release of colored malt compounds into the mash [Lewis&Bamforth, 2006]. This is yet another reason why it is beneficial to mash lighter beers at the lower end of the optimal mash pH range. |
|
conclusionA number of enzymes are active in the mash and many of them have different pH optima which makes it sometimes confusing to choose the proper mash pH. To reduce that confusion, here are some simple guidelines that can be followed when determining which mash pH should be targeted or deciding if the current mash pH needs adjustment:
|
|
Extraction of TanninsTannins are a chemical compound that is found in vegetative matter like grain husks, hops, grape skins and tea leaves. In brewing, the main source of tannins in wort originates from malt husks and hops. If present in excess tannins will give the beer a dry, astringent and sometimes puckering mouthfeel. But why do some beers have a problem with tannins while others don't? This too has to do with pH. The solubility of tannins in water is affected by 2 factors. One of them is temperature and the other one is pH. The effect of temperature is obvious. The higher the temperature the more soluble most compounds including tannins generally are and the higher the rate at which they are leached into the wort. |
|
|
But the effect of pH is more profound. Polyphenols, which tannins are, are weak acids. And as we saw in acids and bases they readily donate protons to their environment if the concentration of H+ is low enough, i.e. the pH is high enough. Once a polyphenol molecule looses one or more protons it becomes negatively charged. Due to the water molecule's charged nature, one side is positive and the other is negative, water is a great solvent for charged molecules and that's why the solubility of tannins increases when pH rises [Bogspot.com, 2009]. A generally accepted pH threshold is 5.8-6.0 [source?]. As a result, the mash pH is generally low enough to prevent excessive tannin extraction. But sparging with high alkalinity water can quickly consume the mash's buffer capacity and lead to pH levels that lead to excessive tannin extraction into the wort. This is because the high concentration of carbonates and bicarbonates in the water forms a strong buffer. As the sweet wort is diluted and with it the mash’s ability to buffer its pH at a level closer to the mash pH, the sparge water and its pH are taking over which can raise the pH above 6.0 and cause excessive tannin extraction. | |
|
There are a number of ways that this pH raise during sparging can be prevented or at least mitigated such that the pH is not allowed to raise above 5.8:
|
|
Protein coagulation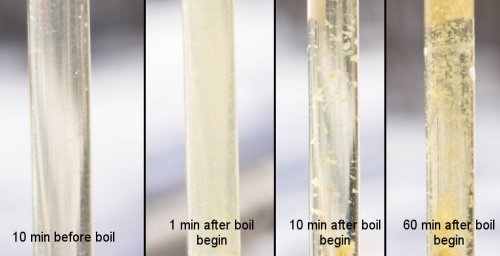 Figure 3 - Wort clarity and break formation before, at the beginning, during, and at the end of the boil. The starting boil pH was 5.35 and the cast out wort pH was 5.30. It can be seen that the wort 10 min before boiling is still clear. At the beginning of the boil protein have coagulated to form a haze. Shortly after that, they started to form flocks (hot break) which cleared up the wort. At the end of the 60 min boil the flocks are larger while the wort is brilliantly clear As we saw with the enzyme activity and the tannin extraction the main effect that a changing pH has is its ability to change the electrical charges on molecules. And that is no different with proteins during wort boiling. But in addition to that, another structural aspect of proteins matters. Proteins are chains of amino acids that are folded into a structure that allows them to do their job in an organism. Some of these amino acids react well with water, they are called hydrophilic, while others try to avoid water and they are called hydrophobic. In water soluble proteins the hydrophobic amino acids are oriented such that they face towards the center of the protein and therefore can react with each other rather than with the surrounding water while the hydrophilic ones face outward. When the protein coagulates its chain of amino acids is not broken but it looses its folded structure and some of the hydrophobic amino acids will face outward. Because hydrophobic amino acids rather react with other hydrophobic amino acids they try to avoid water by joining with hydrophobic amino acids of other coagulated proteins. As a result the proteins clump together and form the flocks we all know as hot and cold break. In addition to that tannins from the husks and hop material, which are present in wort, will attach themselves to these hydrophobic areas. Through hydrogen bonds they will form bridges between coagulated proteins. The aforementioned hydrophobic protein areas are generally rich in the amino acid proline, |
|
|
But how does pH affect this? This is where the concept of the isoelectric point, which we discussed in isoelectric point, plays a role. When the overall charge on the proteins is neutral, which it is at their isoelectric point, they don't repel each other anymore. Such repulsion would be the case at a pH lower or higher than the iso-electric point where the proteins are predominantly negatively or positively charged respectively. When they don't repel each other, hydrophobic sections of the denatured proteins can react with each other more easily and they'll clump together faster. The isoelectric point is also where the solubility of a protein is at its lowest level since uncharged molecules don't react with water as well as charged ones. All this helps protein coagulation during the boil. The iso-electric point of wort proteins is around 4.9 [BrewingTechniques, 1993]. But a common boil pH of 5.2 – 5.4 (room temperature sample) does not reach a pH that low and therefore the protein coagulation is not as good as it could be [Narziss, 2005]. While it is possible to lower the boil pH through the addition of acids, it is generally not done because the level of protein coagulation that is achieved at a pH of 5.2 is sufficient and reduction of the boil pH further reduces the hop alpha acid utilization. During boiling the pH drops by about 0.1 – 0.2 pH units from 5.3 – 5.5 pH to about 5.2 – 5.3 pH. This may be due to the addition of bitter acids from the hops, formation of acidic Maillard products, precipitation of alkaline phosphates or the reaction of polypeptides with calcium, liberating protons [Briggs, 2004]. | |
Hop utilizationBefore hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved into the boiling wort. That solubility depends on both wort temperature and pH. Except for elevation and equipment dependent variations, the boil temperature is largely constant. But boil pH can easily vary and with it the solubility of hop acids. It has been shown that this solubility increases with pH (see Figure 4) [Briggs, 2004] which is why the bitterness extraction from hops is greater at a higher boil pH. Many brewers, however, have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher compared to bitterness gained from a boil at a lower pH. The same mechanism that increases tannin solubility at higher pH increases the solubility of hop resins. Hop resins behave acidic and want to loose H+ at higher pH. That leaves a charged molecule which in turn is more soluble in water. The reduction in solubility of hop acids can be seen during fermentation. After wort is pitched with yeast and the pH falls during fermentation, most of the unisomerized hop resins will come out of solution again. They become part of the brown gunk that floats on top of the Kraeusen which, if a smooth bitterness is desired, should be removed via blow-off tube, skimming and not allowed to fall back into the beer [Narziss, 2005][Kunze, 2007]. |
|
Maillard reactionsMaillard reactions, or non-enzymatic browning, start with a reaction between an amino acid and a reducing sugar. The final products are melanoidins which are responsible for the darkening of both, malt during kilning and wort during boiling. The rate of Maillard reactions is affected by wort concentration, temperature and pH. Here we want to have a look at the effects of pH during the wort boil. The higher pH the faster the rate of Maillard reactions will be. I was able to observe that during my mashing experiments when I boiled the wort from a 5.5 pH and a 6.5 pH mash. The result is shown in Figure 5. While the difference is dramatic, despite the short boil time of only 15 min, a wort boil pH of 6.5 is well out of the normal range. A beer brewed with that of a boil pH will have more severe problems than just increased color. But in general, aiming for a lower boil pH (5.3 - 5.4 for a room temperature sample taken at the beginning of the boil) will keep lighter beers from picking up too much color during boiling. But how is pH affecting the rate of Maillard reactions? It is through its affect on the amino acid. As mentioned earlier, Maillard reactions start with the reaction between a reducing sugar and an amino acid. That reaction yields so called Amadori products which are an important intermediate during the Maillard reaction. This initial reaction happens at a much higher rate when the amino group of the amino acid becomes more likely to donate its electrons (Nucleophilic) to the bond with the reducing sugar. That is the case when the amino group lost a proton (H+) or is on the edge of loosing it to counteract the low H+ concentration (i.e. high pH) of its environment. [khymos.org] In short: high pH -> more amino acids have lost a proton from their amino groups -> they are now more likely to donate an electron to the reducing sugar -> more Amadori products -> more melanoidens. |
|
Nutrient uptake by yeastShortly after being pitched into fresh wort yeast will start lowering the pH of the surrounding medium (i.e. beer). This is the result of ammonium ion and amino acid uptake, secretion of organic acids [Briggs, 2004] and most importantly a proton pump which moves H+ ions from the yeast cell into the beer. By doing so the yeast also raises its internal pH. This proton pump is very important to the yeast and it is the most abundant protein in its cell membrane [Briggs, 2004]. The resulting pH gradient through the yeast's cell wall facilitates the uptake of nutrients like maltose. Maltose uptake is a proton symport process through the cell membrane. The proton concentration outside the cell is greater (lower pH) than inside the cell (higher pH) and therefore a natural gradient exists which encourages protons to flow from the outside to the inside of the cell. Though the use of a symporter, a cell membrane protein, maltose can “piggy back” on the flow of protons into the cell. This is one of the reasons why yeast cells do better in an acidic environment and have means of lowering the pH. |
|
|
The ability of yeast to lower the beer’s pH is important for healthy and low yeast stress fermentation and is one of the reasons why sufficient pitching rates are important and why it is better to step up starters rather than starting a small amount of yeast in a large starter. The more yeast cells that are working on lowering the pH the faster the pH will be able to drop. As yeasts age, starve or otherwise loose their vitality, it becomes increasingly difficult for them to pump H+ from their cells into the beer. After all, this goes against nature’s desire to equalize everything and therefore takes energy. The result is a slight rise of the beer pH after primary fermentation. The pH can rise more significantly if the beer is not taken off the yeast before a large number of yeast cells start to autolyze. |
|
Inhibition of beer spoilage organismsBesides alcohol and hop resins, the low pH of beer, which is generally lower than 4.5, is one of the main reasons why it provides a poor growth medium for many bacteria. There are for example no pathogens (bugs that can make you sick like E. coli or C. botulinum) that can grow in beer and it is therefore safe to even drink beers that have been infected. Wort tends to have a pH between 5.2 and 5.6 which allows the growth of pathogens and care should be taken if it is stored unpitched and unrefrigerated for an extended period of time. The FDA requires that all canned food stuff with a pH greater than 4.6 needs to be heat treated under pressure to allow for temperatures that are high enough for sterilization in order to eliminate pathogens and their spores. That's why wort needs to be pressure canned or frozen to be stored. |
|
Clarifies in kettle and fermenterBrewers may use fining agents in the kettle and in the finished beer. Depending on the product used it aids the precipitation of proteins, yeast cells or tannins but all aim at reducing beer haze and through it improve clarity and haze stability. Here we want to focus on clarifiers that work through electrostatic forces. This is the case for collagen's (Gelatin or Isinglass) affect on yeast cells and to some extend the case for carrageen's (Irish Moss or Whirlfloc) affect on wort proteins. Other clarifiers like silica gel and tannic acid bind with proteins through reactions with their hydrophobic regions. Collagen is a very effective clarifier which is used after fermentation is complete. It is the effective compound in isinglass and gelatin. Collagen is a protein that, depending on its origin and processing has an isoelectic point of ~5.5 (isinglass) [Alton], 7.0 – 9.0 (type A gelatin) or 4.7-5.4 (type B gelatin) [GMIA]. Isinglass is produced from the swim bladder of tropical fish; gelatin on the other hand is either made from pig skin and feet (type A) or bovine (cow) parts (type B). For all of these sources of collagen the IEP (isoelectric point) is above the pH of beer which means that the collagen molecules carry a net positive charge. That was shown in Isoelectric point. Yeas cells, however, are negatively charged. After all, they actively pump protons, which carry a positive charge, into their environment. The different polarity of their electrical charges cause the yeast to be attracted to the collagen molecules. Due to its molecule size the collagen slowly settles to the bottom of the vessel and takes the yeast with it. Gelatin and isinglass are not only known to aid yeast settlement but remove protein hazes as well. Proteins in beer are largely positively charged (the same charge as the collagen) and gelatin and isinglass will show little to no effect on the protein part of beer hazes. However, protein haze is formed by bonds between tannins and protein and it is the tannins that collagen bonds with. When it then settles to the bottom it takes the protein-tannin complexes, which cause the haze, with it. Because of its wide availability, almost every grocery store carries it, most brewers use Knox unflavored gelatin if they need to clarify a beer. Knox gelatin is made from pork and therefore type A [Kraftfoods]. Whenever I use it I use about 3-4 g (½ packets) dissolved in 100-200 ml warm water for a 16-20 l (4-5 gal) batch of beer. The water should be sanitary and sanitary procedures need to be followed since the gelatin suspension cannot be boiled. Once the gelatin is completely dissolved, the mix is added to the beer and mixed in well. It should take only a few days for the gelatin to settle after which the clear beer can be drawn off. Clarifiers can also be used in the boil kettle. Popular products are Irish Moss and Whirlfloc. The active ingredient in both is carrageen. In contrast to collagen carrageen is a polysaccharide that is strongly negatively charged over the pH range encountered in brewing [Martin, 2000]. But at boil pH most proteins are at or slightly above their isoelectric point which means they carry a neutral or slightly negative charge and should therefore not be attracted to carrageen. In fact the use of carrageen (e.g. Irish Moss) in the boil does not increase the protein precipitation but it causes the formation of larger flocks that settle more quickly and form better trub cones in the whirlpool [Lewis&Bamforth, 2006]. The use of Irish Moss or Whirlfloc is not permitted by the German purity law. Neither is gelatin, but isinglass can be used if the beer is subsequently filtered [Narziss, 2005]. | |
Beer tasteLast but not least the pH of beer also affects its taste perception. A low beer pH results in a crisper more lively beer while a high beer pH is generally associated with a dull flavor perception. But there are limits to how low pH can be before the beer's taste starts to take on sour notes. For all malt beers a pH range of 4.25 - 4.6 [Narziss, 2005] is generally accepted as optimal while adjunct beers can be as low as 4.0 [Kunze, 2007] and sour beers will be even lower. The lower pH of adjunct beers is a result of the lower buffer capacity of the adjuncts. In this case yeast is able to lower the pH further because the beer does not offer as much resistance (buffering) as it does in all malt beers. How pH changes the taste becomes evident if the taste of a post boil wort sample and that of very young beer is compared. At the beginning of fermentation yeast lowers the pH while no or only little fermentation has happened yet. As a result the sample with yeast will taste sweeter and fresher than the post boil sample which tastes more dull even though they both have a nearly identical sugar profile. |
|
References
|