Difference between revisions of "Starch Conversion"
(→Effect of mashing conditions) |
(→Effect of mashing conditions) |
||
| Line 52: | Line 52: | ||
| − | {| class="ikitable" border=" | + | {| class="ikitable" border="0" alight="right" |
|+Multiplication table | |+Multiplication table | ||
|- | |- | ||
Revision as of 17:00, 16 January 2009
96% (?) of the solids in brewing wort are carbohydrates. Proteins make up only 3% and the rest are β-glucans, vitamins and minerals. Of the carbohydrates more than 95% are products of the starch conversion that happens in the mash tun. As a result the starch degradation is the main purpose of mashing. It produces fuel that the yeast needs for fermentation from the insoluble starch found in malt and mash tun adjuncts. The extend to which this conversion happens will determine the strength (gravity or extract content) of the produced wort and its fermentability as well as other quality parameteres.
In mashing starch conversion is preceded by gelatinization of the starches. While this is not necessary for conversion, as plants do exactly this when they germinate, it greatly speeds up the process though the exposure of a lot of substrate (amylose and amylopectin) from the starch granule to the starch converting enzymes (mainly α- and β-amylase).
Contents
Gelatinization
Starch granules are insoluble in cold water and will absorb only little water. The form a suspension that quickly settles once agitation stops. As the water is heated (50+ C) more and more water is absorbed and the granules start to swell [Narziss, 2005]. The water absorbed during this process can be up to 30 times the weight of the starch granule. This uptake of water initially happens within the amorphous growth rings. The granule starch to leach amylose and the crystalline layers break open and separate from the starch granule as gelatenous sheets. At this point the crystalline structure is lost and the process becomes irreversible with respect to the shape of the starch granule [Shetty, 2006]. The starch granule has gelatinized.
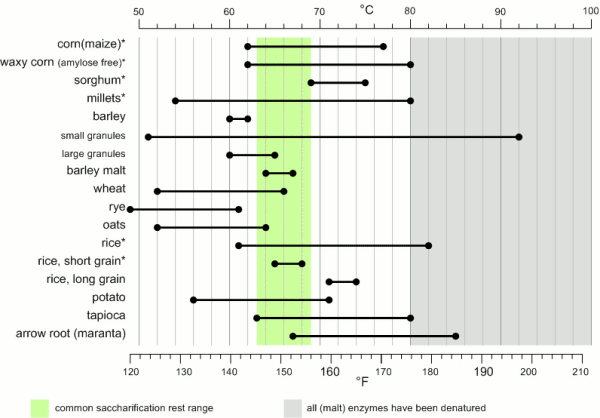
While the temperature range during with gelatinization occurs has been found to be quite narrow for individual starch granules (~ 1C) the temperature range between the gelatinization of the first granules and the complete gelatinization of all granules can be quite large. Figure 1 shows the temperature ranges for gelatinization for a number of starches. It can be seen that not all these starches fully gelatinize at temperatures that are encountered in a saccharification rest. If this is not the case they will have to be gelatenized before that rest though either a cereal mash or the use of pre-gelatinized (e.g. flaked) forms. Another interesting aspect is the different gelatinization temperature ranges that have been determined for large and small barley granules. 90% of the starch in barley are large granules which will be gelatenized at saccharification rest temperatures while the rest are small starch granules which may not fully gelatenize until higher temperatures are reached. This effect can explain some of the efficiency benefits that can be gained from a mash-out or a decoction mash.
Gelatinization is a process that requires free water for the swelling and breaking the hydrogen bonds that hold the crystalline structures in place. If free water is limited due to a high concentration of starch (e.g. overly thick mash conditions) less swelling takes place and a melting of the crystalline section needs to occur [Donald, 2004]. This leads to an increase in the gelatinization temperature. This limitation of free water can also be caused by the presence of sugars other dissolved solids. For corn starch it has been shown that a 25% sucrose solution increase the gelatinization temperature from 70 to 78C [Donald, 2004]. This could have been one of the factors why thick mashes showed a lower efficiency compared to thin mashes in the Mashing Experiments.
As starch starts to gelatenize the viscosity of the liquid will increase. This can be noticed in brewing to some extend but by far less that what is commonly seen in cooking. The reason for that is the presence of enzymes (in particular α-amylase) that will start breaking down the amylose and amylopectin molecules as soon as they become accessible. This process reduces the viscosity of the mash and is therefore called liquification [Kunze, 2007]. Liquifying decoctions through a rest at 70-74C before further heating to a boil for example mitigates the risk of scorching the mash during the decoction boil.
A strong increase in viscosity can however become a problem in cereal mashes. Especially when using rice starch which is known to swell very strongly. This can lead to scorching or even the immobilization of mash agitators [Kunze, 2007]. To counteract that some malt should be added to cereal mashes and a short liquification rest might be held between 75 and 80C (just before all the α-amylase gets denatured) before it is then heated to boiling.
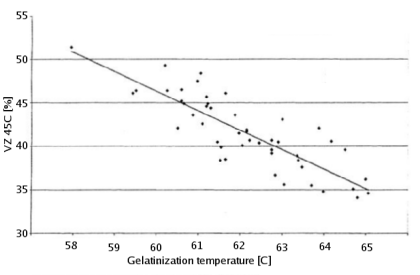
The gelatinization temperature also depends on growing conditions and crop year [Kunze, 2007]. And Kessler showed that a weak correlation exists between the VZ 45C malt analysis number and the gelatinization temperature [Kessler, 2008]. VZ 45C is the ratio between the extract that can be extracted through mashing at 45C and the amount that can be extracted with a congress mash. This number is given on some malt analysis sheets. Figure 2 shows the data that was publushed in Brauwelt International. According to Weyermann the VZ45 for their malts can be as low as 35. This may result in a gelatinization temperature as high as 65C. While this temperature should not be a problem when using a single saccharification rest, it can become problematic when a maltose rest is held at 63C at which temp the starch will not be fully gelatenized. If this is the case an extended rest at 65C needs to be held in order to achieve the desired fermenntability of the produced wort.
Enzymatic starch breakdown
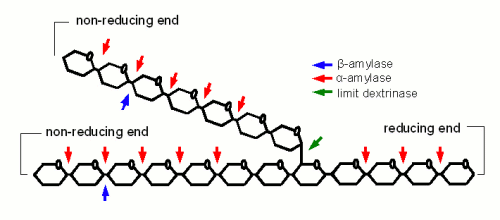
Starch conversion in the mash is mainly an enzymatic process and there are 4 types of enzymes that can take part. The 2 best known enzymes are α- and β-amylase. Both work on the α(1-4) glycosidic bonds of the starch and dextrin molecules. Another enzyme is limit dextrinase which is able to break the α(1-6) glycosidic bond that forms the branch points in the amylose molecule. At last there is maltase, an enzyme that can break maltose into two glucose molecules. But because this enzyme is already deactivated by temperatures above 45 C, it generally doesn't play any significant role in mashing.
α-amylase is an enzyme that can cleave any α(1-4) bond in starch and dextrines as except for the ones close to the branch points. This breakage of a glycosidic bond is also called hydrolyzation as it consumes one water molecule. It is the enzyme that is responsible for the rapid loss of viscosity after malt starch gelatinized. A process that is called liquefaction of the mash. While α-amylase is very good at breaking down starch into smaller dextrines, which quickly reduces the iodine reaction from a black-blue to a more reddisch brown color, it is not very good at producing fermentable sugars. This results from the low affinity that α-amylase has towards smaller glucose chains.
β-amylase is much better at producing fermentable sugars. In fact the way in which β-amylase hydrolyzes starches and large dextrines is the reason for the large maltose content in wort produces through mashing. It attaches to the non-reducing end of a glucose chain and clips off one maltose molecule after another. But because it has to do that at the non-reducing end it’s activity has to stop when it comes close to a branch point (α(1-6) link). In order to reach the glucose chains after the branch points it relies on the activity of the α-amylase which creates a non-reducing end every time it splits a glucose chain or the limit dextinase which can break the branching α(1-6) link itself. In mashing α- and β-amylase work in concert: α-amylase creates substrate (i.e. non-reducing ends) for β-amylase. The process of creating sugars is called saccharification and happens mostly after the liquefication of the mash. Mashed that intend to produce a highly fermentable wort always try to maximize the β-amylase activity. This is mainly done through the rest tempearature(s) and the time spent at these rest(s) which will be discussed later
α- and β-amylase alone cannot completely saccharify (i.e. convert to all sugar) starch. The reason for that are α(1-6) that make up 6-7% of the bonds in amylopectin. But malt contains one enzyme that can: limit dextrinase. But the problem with limit dextrinase is that is not as heat stable as α- and β-amylase and quickly denatures at temperatures above 60C. Since at this temperature only little starch may be gelatinized and accessible yet there aren’t many branch points for this enzyme to work on. As a result most brewing wort will contain a large number of limit dextrines which are the branch points that were left behind by α- and β-amylase activity. Unless a very high fermentability is desired the residual dextrines are actually desired as they contribute positively to the character of the beer.
Maltase is an enzyme that has its optimum between 35 and 40 C and produces 2 glucose molecules from one maltose molecule. It is generally of little interest in mashing as at its working temperature there is not much maltose present in the wort (given that the mash is doughed in at or below 40 C). If activity of this enzyme is desired to increase the glucose level of the wort the mash needs to be held for saccharification at 63-65C and after having been cooled to 40 C fresh malt is added which also adds new maltase enzymes. After a rest of 30-45 min the mash is heated again to convert the starch that has been added with the new malt. This mash schedule has been introduced by Markus Hermann from the Weihenstephan brewing school to produce high glucose worts for ester rich Weissbiers [Hermann, 2005]
The consumption of one water molecule means that the production of 1 kg maltose (or an equivalent mix of glucose, maltose and larger sugars/dextrines) from starch consumes about 50 ml of water. A typical mash for a 19 liter (5 gal) batch that contains about 3 kg of starch which is converted to mostly fermentable sugars will consume about 150 ml water. This is in the order of 1% of the water added to the mash and generally neglected when calculating the amount of water needed for brewing.
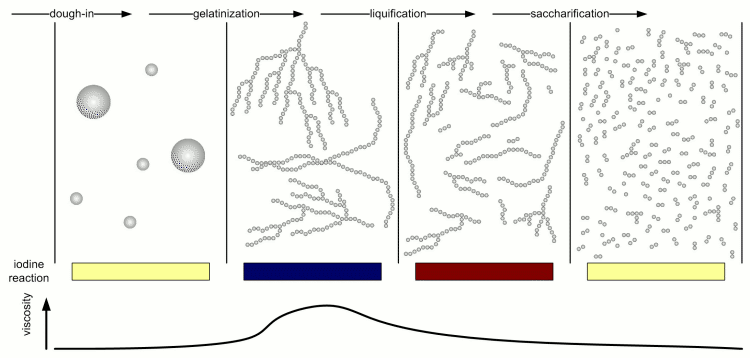
Kunze describes 4 different stages of starch conversion in the mash [Kunze, 2007] (Figure 4). Assuming a low enough dough-in temperature after dough in the starch is present as a suspension of starch granules in water. Once agitation is stopped these starch granules quickly settle. No iodine reaction can be detected yet and the viscosity of the liquid is low. But Amylase enzymes may already attack the starch granules and free and convert some starch. As the temperature is increased the gelatinization temperature of the starch is reached and the granules release their amylose and amylopectin. These large molecules immediately thicken the mash and its viscosity rises sharply. The iodine reaction will be blue-black at this stage. But at this point the amylase (especially the α-amylase) and limit dextrinase enzymes will quickly break the large amylose and amylopectin molecules into large dextrins which greatly reduces the viscosity of the mash. The brewer calls this liquification of the mash. The iodine reaction becomes less blue and more reddish brown as an indication that the size of the glucose chains is reduced. As the dextrines become smaller β-amylase and limit dextrinase become more important in efficienctly reducing them to mostly fermentable sugars as the α-amylase only shows a low affinity towards these smaller dextrines. This stage is called saccharification and is completed when the iodine completely disappears.
Effect of mashing conditions
| × | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 1 | 2 | 3 |
| 2 | 2 | 4 | 6 |
| 3 | 3 | 6 | 9 |
| 4 | 4 | 8 | 12 |
| 5 | 5 | 10 | 15 |
[Briggs, 2004]:
- gelatinization
- active enzymes
- emzyme temperature and pH optima
- mash parameters affecting conversion
References
- [Valclavik] Vickie A. Valclavik, Elizabeth W. Christian, Essentials of Food Science, Third Edition, Springer
- [Champe] Pamela C, Champe, Richard A. Harvey, Denise R. Ferrier, Biochemistry, Lippincott's Illustrated Reviews
- [Kunze, 2007] Wolfgang Kunze, Technologie Brauer und Maelzer, 9. Auflage, VLB Berlin
- [Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, Abriss der Bierbrauerei, Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan). WILEY-VCH Verlags GmbH Weinheim Germany, 2005
- [Donald, 2004] A. M. Donald, Understanding Starch Stucture and Functionality, Chapter 5 in Starch in Food: Structure, Function and Applications By Ann-Charlotte Eliasson, CRC Press, 2004
- [Shetty, 2006] Kalidas Shetty, Food Biotechnology, CRC Press, 2006
- [Kessler, 2008] Dr.-Ing. Matthias Kessler, Dr.-Ing. Stefan Kreisz. Dipl.-Ing. Martin Zarnkov. Univ.-Prof. Dr.-Ing. Werner Back, Do Brewers need a starch modification index?, Brauwelt International, 2008/1