Residual Alkalinity illustrated
|
This used to be the “Understanding Mash pH” article. But over time some of the article’s contend had become outdated as I gained a much better understanding of mash pH. A 3 part series was written to replace that article:
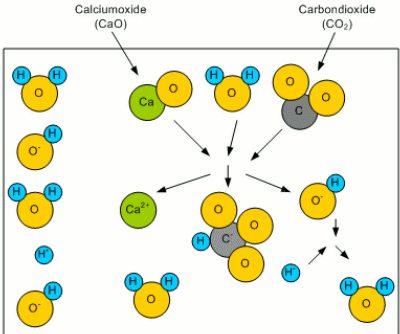
What remains of the old article is a series of illustrations that visually explain the concept of residual alkalinity. AlkalinityWhen this water rains down from the atmosphere, it picks up CO2 to form carbonoic acid. Even more CO2 is picked up while the water trickles through the soil. This carbonic acid is then able to react with minerals like calcium to form calcium oxide: CaO + H2O + CO2O -> Ca2+ + HCO3- + HO- The result of this reaction are a bicarbonate (HCO3-) and a hydroxide (HO-) ion. Both of which can bind a proton (H+) and thus reduce the number of free protons. This results in an increased pH (less acidic) as well as in an increased buffering capacity. The latter is the ability of the solution to resist a change of pH (concentration of protons) even if additional protons are added (e.g. through the addition of an acid). The bicarbonate ion (HCO3-) reacts with the added protons (H+): HCO3- + H+ -> H2O + CO2O Forming water (H2O) and carbon dioxide (CO2O). This buffering capacity is called the alkalinity of the water and can be expressed as either ppm HCO3 or ppm CaCO3. The latter is an equivalent concentration of dissolved chalk (CaCO3) that hat provides the same alkalinity.
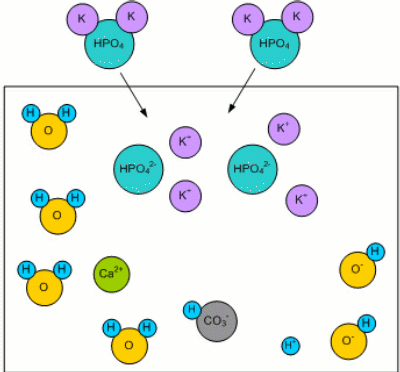
Adding MaltWhen malt is added, the malt's phosphates (mainly potassium phosphate K2HPO4 [Fix,1999]) dissolve in the mash water: K2HPO44 -> 2K+ + HPO42-
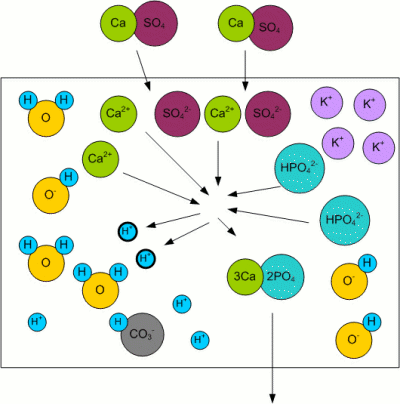
Residual AlkalinityKolbach, a German brewing scientist, found that the malt's phosphates react with the calcium and magnesium ions from the mash water [Fix, 1999]: 3Ca2+ + 2HPO42- <-> 2H+ + Ca3(PO4)2 This reaction releases 2 protons (H+) as well as calcium phosphate (Ca3(PO4)2) which is pretty much insoluble in wort and precipitates. The important aspect however is the release of 2 protons which can react with the bicarbonate ions (HCO3-) that are responsible for the water's alkalinity [Narziss, 2005]: H+ + HCO3- -> H2O + CO2 The result is a lowered alkalinity or buffering capacity of the water. If no more bicarbonate ions are present, the pH sinks due to the increase of protons (H+). Kolbach's work found that not all of the water's calcium and magnesium ions take part in the aforementioned reaction. He found that only 2 out of 7 calcium ions and one out of 7 magnesium ions react with the malt's phosphates to release protons. This resulted in the definition of residual alkalinity, which is a measure of the alkalinity left after the acidifying reaction between the malt's phosphates and the water's calcium and magnesium has been taken into account: When the calcium, magnesium and bicarbonate concentrations can be expressed in an unit that is equivalent to the ion concentration (as opposed to the weight concentration) of these ions (like dH (German Hardness) or mEq/l), the equation for the residual alkalinity simply is: RA = KH - (CH + 0.5 * MH)/3.5 Where RA is the residual alkalinity in dH, KH the alkalinity (carbonate hardness), CH the Calcium Hardness and MH the Magnesium Hardness. But a water report usually doesn't show these parameters as dH, especially outside of Germany. In order to convert this formula such that it can be used with ppm as the unit, the molar weight of the carbonate, calcium and magnesium ions has to be taken into account. Though this is straight forward, it involves juggling a lot of numbers and since it doesn't help in understanding water chemistry i created a spread sheet for making these calculations: Kaiser_water_calculator.xls |