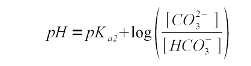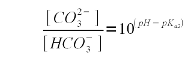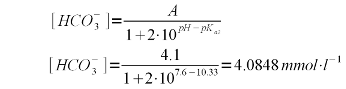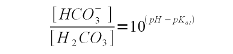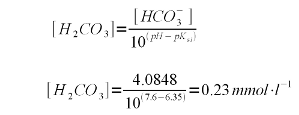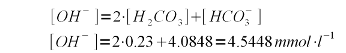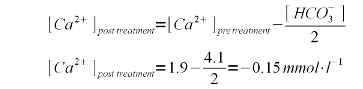Difference between revisions of "Alkalinity reduction with slaked lime"
m (→Resulting water profile) |
|||
| (42 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
| | | | ||
| − | + | High alkalinity in their brewing water keeps many home brewers from being able to brew lighter beers well. To overcome this obstacle many of them use reverse osmosis water to dilute their water or even build their brewing water from scratch through the addition of salts. Another option is the use of acids to neutralize some or all of the water's alkalinity. | |
| − | + | If most or all of the water's alkalinity is the result of temporary hardness (dissolved calcium and magnesium carbonate) another options exists which is commonly overlooked: alkalinity precipitation using calcium hydroxide (also known as slaked lime). Due to its low cost this method of water treatment is widely used in the brewing industry but home brewers appear to have largely stayed away from it due a lack of understanding of the chemistry behind it and missing support in brewing water calculators. | |
| − | + | Based on A.J. deLange's work <ref name="deLange">A.J. deLange, [http://ajdel.wetnewf.org:81/Brewing_articles/Cerevesia/Final_galley Alkalinity, Hardness, Residual Alkalinity and Malt Phosphate: Factors in the Establishment of Mash pH], 2004</ref> and through pointers in technical brewing literature <ref name="Narziss">Ludwig Narziss, Werner Back, Die Bierbrauerei Band 2: Technologie der Würzebereitung, Wiley-VCH, 2009</ref> I was able to get it to work with my water and add support for the necessary calculations to the water calculation spreadsheet ([http://braukaiser.com/documents/Kaiser_water_calculator.xls Kaiser_water_calculator.xls]). The following article attempts to shed light on this process and provide a better understanding of the chemistry behind it which hopefully allows other brewers to use this elegant form of water treatment in their brewing process. | |
| − | + | ||
| − | Based on A.J. deLange's work | + | |
=An unintuitive concept explained= | =An unintuitive concept explained= | ||
| Line 15: | Line 13: | ||
| | | | ||
| − | + | Most brewing books mention that the alkalinity and hardness of brewing water can be lowered through the addition of a lime (calcium hydroxide) which itself is a strong alkali. ''How can the addition of a strongly alkaline compound like slaked like reduce alkalinity?'' This always confused me and it took me a while until the chemistry behind this process became clear. | |
| − | + | ||
| − | Most brewing books mention that the alkalinity and hardness of brewing water can be lowered through the addition of a lime (calcium hydroxide) which itself is a strong alkali. ''How can the addition of a strongly alkaline compound like | + | |
To better understand the chemical process described here the reader should have read [[An Overview of pH]] as well as [[Building brewing water with dissolved chalk#About chalk and the carbonate system|About chalk and the carbonate system]]. | To better understand the chemical process described here the reader should have read [[An Overview of pH]] as well as [[Building brewing water with dissolved chalk#About chalk and the carbonate system|About chalk and the carbonate system]]. | ||
| Line 26: | Line 22: | ||
The resulting hydroxide ions (OH<sup>-</sup>) want to raise the pH of the solution and in pure water will be able to that very effectively. However, the water we are treating with lime contains bicarbonate and carbonic acid with acts as a pH buffer and by doing so stand in the way of a large pH rise. While we have experienced alkalinity only as a hindrance for lowering the pH of the mash a pH buffer works both ways. I.e. it keeps the pH from falling as much as as it keeps it from rising. | The resulting hydroxide ions (OH<sup>-</sup>) want to raise the pH of the solution and in pure water will be able to that very effectively. However, the water we are treating with lime contains bicarbonate and carbonic acid with acts as a pH buffer and by doing so stand in the way of a large pH rise. While we have experienced alkalinity only as a hindrance for lowering the pH of the mash a pH buffer works both ways. I.e. it keeps the pH from falling as much as as it keeps it from rising. | ||
| + | |||
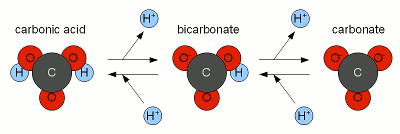
| + | [[Image:Carbonic_acid_dissociation.gif|frame|center|'''Figure 1''' - The relative concentration of carbonic acid, bicarbonate and carbonate based on the pH of the solution. The EPA (Environmental Protection Agency) recommends drinking water to have a pH between 6.5 and 8.5 <ref name="EPA">epa.gov, [http://www.epa.gov/ogwdw000/consumer/2ndstandards.html Secondary Drinking Water Regulations]</ref>]] | ||
[[Image:Carbonic_acid_to_carbonate.gif|frame|right|'''Figure 2''' - The transformation of carbonic acid to carbonate over bicarbonate and back to carbonic acid]] | [[Image:Carbonic_acid_to_carbonate.gif|frame|right|'''Figure 2''' - The transformation of carbonic acid to carbonate over bicarbonate and back to carbonic acid]] | ||
| Line 53: | Line 51: | ||
=Limitations= | =Limitations= | ||
| − | This form of water treatment can only reduce alkalinity if sufficient calcium is present since it relies on the formation of calcium carbonate. Though changes can be made to the procedure (see [[#Reducing magnesium hardness|Reducing magnesium hardness]]) the amount of magnesium that can be removed with this method is limited due to the fact that magnesium carbonate is about 100 times more soluble than calcium carbonate [uri.edu]. | + | This form of water treatment can only reduce alkalinity if sufficient calcium is present since it relies on the formation of calcium carbonate. Though changes can be made to the procedure (see [[#Reducing magnesium hardness|Reducing magnesium hardness]]) the amount of magnesium that can be removed with this method is limited due to the fact that magnesium carbonate is about 100 times more soluble than calcium carbonate<ref name="uri">[http://bilbo.chm.uri.edu/CHM112/tables/KspTable.htm Solubility Product Constants near 25 C], University of Rhode Island</ref>. |
Sodium cannot be reduced with this method since sodium carbonate is very soluble in water. What cannot be reduced either are sulfate and chloride anions. Those will remain unchanged unless more are added though the addition of calcium salts, a practice that is commonly used to boost the water's calcium content and ensure an adequate calcium concentration in the treated water. | Sodium cannot be reduced with this method since sodium carbonate is very soluble in water. What cannot be reduced either are sulfate and chloride anions. Those will remain unchanged unless more are added though the addition of calcium salts, a practice that is commonly used to boost the water's calcium content and ensure an adequate calcium concentration in the treated water. | ||
| Line 65: | Line 63: | ||
Because of the precipitation of calcium it might be necessary to supplement the water with calcium using calcium carbonate or gypsum. This is best done before or together with the lime addition. The amount needed depends on the expected chalk precipitation and the targeted calcium level of the post-treatment water. | Because of the precipitation of calcium it might be necessary to supplement the water with calcium using calcium carbonate or gypsum. This is best done before or together with the lime addition. The amount needed depends on the expected chalk precipitation and the targeted calcium level of the post-treatment water. | ||
| − | All of the calcium, which is added by the lime, will also be precipitated in the form of calcium carbonate. A fact that provides compliance with the German ''Reinheitsgebot'' since in the end nothing is added to the water | + | All of the calcium, which is added by the lime, will also be precipitated in the form of calcium carbonate. A fact that provides compliance with the German ''Reinheitsgebot'' since in the end nothing is added to the water <ref name="Narziss"/>. In practice this method is not able to completely reduce the alkalinity to 0 regardless of the amount of calcium that is present. The practical lower limit of alkalinity reduction is 30-50 ppm as CaCO<sub>3</sub>. One reason for that is absorption of atmospheric CO2 during the settling time which causes some of the precipitating chalk to be dissolved again. |
| − | While the reaction also takes place at low water temperatures the precipitating calcium carbonate forms larger particles in slightly warmer water and as a result the temperature of the treated water should not be below 12 C (54 F) | + | While the reaction also takes place at low water temperatures the precipitating calcium carbonate forms larger particles in slightly warmer water and as a result the temperature of the treated water should not be below 12 C (54 F) <ref name="Narziss"/> |
| valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| Line 85: | Line 83: | ||
== Lime needed and calcium balance == | == Lime needed and calcium balance == | ||
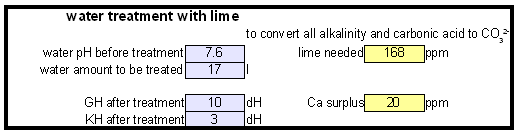
| − | Now jump ahead to the "water treatment with lime" section. Here the current water pH and the water amount to be treated need to be specified. The water pH is used to determine how much carbonic acid and CO<sub>2</sub> are present in the water. This is necessary since the added lime also needs to convert that to carbonate in addition to converting the bicarbonate to carbonate. The water amount to be treated is different from the total brewing water amount specified later since some water is left behind to | + | Now jump ahead to the "water treatment with lime" section. Here the current water pH and the water amount to be treated need to be specified. The water pH is used to determine how much carbonic acid and CO<sub>2</sub> are present in the water. This is necessary since the added lime also needs to convert that to carbonate in addition to converting the bicarbonate to carbonate. The water amount to be treated is different from the total brewing water amount specified later since some water is left behind to separate the precipitate from the treated water. |
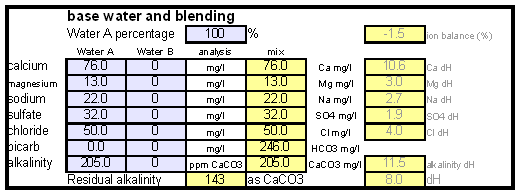
The result of the calculation is the theoretical lime amount, as ppm or mg/l, that needs to be added to precipitate all the water's alkalinity. But the more important result that needs attention here is the calcium surplus. This is the amount of calcium that would remain in the water if all alkalinity is precipitated with this process. If that concentration is below 10 ppm you need to add some calcium salts (Gypsum or Calcium Chloride) to boost the calcium content of the water. In my case I had to do that which will be explained in the next section. | The result of the calculation is the theoretical lime amount, as ppm or mg/l, that needs to be added to precipitate all the water's alkalinity. But the more important result that needs attention here is the calcium surplus. This is the amount of calcium that would remain in the water if all alkalinity is precipitated with this process. If that concentration is below 10 ppm you need to add some calcium salts (Gypsum or Calcium Chloride) to boost the calcium content of the water. In my case I had to do that which will be explained in the next section. | ||
| Line 99: | Line 97: | ||
Jumping back to the "water modification using salts" section. | Jumping back to the "water modification using salts" section. | ||
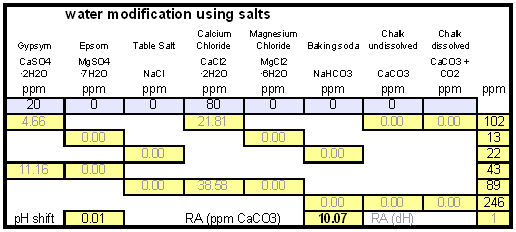
| − | As mentioned earlier, | + | As mentioned earlier, the water used here contains more alkalinity than calcium hardness which limits the efficiency of the alkalinity removal and causes a calcium deficiency in the final water. To avoid this I decided to boost the water's calcium level with 20 ppm gypsum and 80 ppm calcium chloride. By doing so the calcium, sulfate and chloride levels of the water were raised. Their new concentrations in the pre-treatment water can be seen on the right most column. |
[[Image:Lime_treatment_calc_salts.gif|center]] | [[Image:Lime_treatment_calc_salts.gif|center]] | ||
| Line 105: | Line 103: | ||
== Salt and lime amounts needs for water treatment == | == Salt and lime amounts needs for water treatment == | ||
| − | When treating water with lime the necessary salts and lime are added to a water amount that is larger than what is needed for brewing. That's why there is a | + | When treating water with lime the necessary salts and lime are added to a water amount that is larger than what is needed for brewing. That's why there is a separate section labeled "necessary salt additions for lime treatment" which calculates the salt amounts based on the salt and lime concentrations needed well as the water amount specified earlier. |
It is unlikely that baking soda is added to the water when alkalinity removal is the goal. Though chalk will be added as well it is only added as a process aid and will not be carried over into the post-treatment water. This is why the list of salts is limited to gypsum, Epsom salt, table salt, calcium chloride and magnesium chloride. The amount of lime needed is given both as the amount of the dry power or a 5% (by weight) solution. The latter is useful for the treatment of small water amounts for test batches which will be explained later. | It is unlikely that baking soda is added to the water when alkalinity removal is the goal. Though chalk will be added as well it is only added as a process aid and will not be carried over into the post-treatment water. This is why the list of salts is limited to gypsum, Epsom salt, table salt, calcium chloride and magnesium chloride. The amount of lime needed is given both as the amount of the dry power or a 5% (by weight) solution. The latter is useful for the treatment of small water amounts for test batches which will be explained later. | ||
| Line 121: | Line 119: | ||
==Mash pH estimation== | ==Mash pH estimation== | ||
| − | After strike water, grist weight and beer color have been specified the mash pH can be estimated. In my case a 11 SRM Maibock was brewed and the estimated mash pH of 5.4 was close enough to the actual mash pH of 5.5. The pH estimation is less accurate when most of the color is coming from base malts which was the case for that grist | + | After strike water, grist weight and beer color have been specified the mash pH can be estimated. In my case a 11 SRM Maibock was brewed and the estimated mash pH of 5.4 was close enough to the actual mash pH of 5.5. The [[Beer color, alkalinity and mash pH|pH estimation]] is less accurate when most of the color is coming from base malts which was the case for that grist |
[[Image:Lime_treatment_calc_beer_info.gif|center]] | [[Image:Lime_treatment_calc_beer_info.gif|center]] | ||
| Line 129: | Line 127: | ||
To efficiently treat water with lime you'll need a few supplies, some of which you may already have available. | To efficiently treat water with lime you'll need a few supplies, some of which you may already have available. | ||
| − | Food grade '''calcium hydroxide''', a.k.a. | + | Food grade '''calcium hydroxide''', a.k.a. slaked lime or hydrated lime, can be found in the canning section of many grocery stores under the name pickling lime. If your local stores don't carry it you can always order it from on-line merchants. A 1lb bag will last a long time. |
{| | {| | ||
|| [[Image:Warning_100x100.png]] | || [[Image:Warning_100x100.png]] | ||
| − | || Calcium hydroxide, also known as hydrated or | + | || Calcium hydroxide, also known as hydrated or slaked lime, is an aggressive substance and should be handled with care. In combination with water, even moisture from the skin or eyes, it forms a caustic solution that can cause chemical burns. Keep it out of reach of children and pets. In addition to that wear safety glasses and gloves whenever you are using it. Spills should be cleaned immediately. Please review this material safety data sheet for slaked lime [http://www.jtbaker.com/msds/englishhtml/C0407.htm Hydrated Lime MSDS] for possible dangers and first aid. |
|} | |} | ||
| Line 151: | Line 149: | ||
|- | |- | ||
| valign="top" | [[Image:Lime_chemicals.jpg|frame|'''Figure 2''' - Weigh out the amounts of salt and lime determined by the | | valign="top" | [[Image:Lime_chemicals.jpg|frame|'''Figure 2''' - Weigh out the amounts of salt and lime determined by the | ||
| − | spreadsheet. In addition to that add about 1/4 - 1/2 tsp of chalk for every 20 l (5 gal ) of | + | spreadsheet. In addition to that add about 1/4 - 1/2 tsp of chalk for every 20 l (5 gal ) of treated water. There is no need to |
| − | be precise with the chalk since its only purpose is to provide | + | be precise with the chalk since its only purpose is to provide crystallization sites for the precipitating chalk. The addition of |
chalk is not necessary but helpful]] | chalk is not necessary but helpful]] | ||
| valign="top" | [[Image:Lime_tapwater.jpg|frame|'''Figure 3''' - Fill a large vessel with the water to be treated. For | | valign="top" | [[Image:Lime_tapwater.jpg|frame|'''Figure 3''' - Fill a large vessel with the water to be treated. For | ||
| − | illustration | + | illustration purposes I used a carboy but since its capacity tends to be less than the amount of brewing water needed it may not |
be the best choice. It also simplifies siphoning or draining the post treatment water if the vessel is placed in an elevated | be the best choice. It also simplifies siphoning or draining the post treatment water if the vessel is placed in an elevated | ||
position.]] | position.]] | ||
| Line 162: | Line 160: | ||
{| | {| | ||
|- | |- | ||
| − | | valign="top" | [[Image:Lime_adding_salts.jpg|frame|'''Figure 4''' - Add the salts to the water]] | + | | valign="top" | [[Image:Lime_adding_salts.jpg|frame|'''Figure 4''' - Add the salts, lime and chalk to the water]] |
| − | | valign="top" | [[Image:Lime_mixing.jpg|frame|'''Figure 5''' - using a mixer, mash paddle or just a large spoon mix water, salts, lime and chalk well.]] | + | | valign="top" | [[Image:Lime_mixing.jpg|frame|'''Figure 5''' - using a mixer, mash paddle or just a large spoon mix water, salts, lime and chalk well. Though the water should be mixed in well, the mixing action should minimize the uptake of atmospheric carbon dioxide since it counteracts the pH rising reaction of the lime]] |
| valign="top" | [[Image:Lime_start_pH.jpg|frame|'''Figure 6'''- If you have a pH meter or pH test strips with a range of 8 - 11 | | valign="top" | [[Image:Lime_start_pH.jpg|frame|'''Figure 6'''- If you have a pH meter or pH test strips with a range of 8 - 11 | ||
you can test the pH after the lime addition. It should be well above 9 but less then 11.]] | you can test the pH after the lime addition. It should be well above 9 but less then 11.]] | ||
| Line 183: | Line 181: | ||
| | | | ||
| − | = | + | = experimenting with the amount of lime actually needed = |
[[Image:Lime_treatment_experiments.gif|right|frame|Figure 10 - The alkalinity and pH of water treated with lime. The x-axis represents the lime added as percent of the calculated concentration]] | [[Image:Lime_treatment_experiments.gif|right|frame|Figure 10 - The alkalinity and pH of water treated with lime. The x-axis represents the lime added as percent of the calculated concentration]] | ||
| − | Through experiments I found that the amount of lime calculated by the spread sheet is not necessarily the optimal amount that gives the best alkalinity reduction. This might be the result of inaccuracies in the water analysis data and simplifications that were made for calculations. As a result it is recommended to test the lime amounts in a small series of experiments | + | Through experiments I found that the amount of lime calculated by the spread sheet is not necessarily the optimal amount that gives the best alkalinity reduction. This might be the result of inaccuracies in the water analysis data and simplifications that were made for the calculations. As a result it is recommended to test the lime amounts in a small series of experiments where varying amounts of lime are added to the water and the alkalinity of the resulting water is tested. |
Conducting these experiments is fairly straight forward. Take the pre-treatment water and add salts to match the concentration of salts that you plan to your brewing water. In addition to these salts add some chalk to the water. This chalk will not dissolve but provides nucleation sites for the chalk that will precipitate from the water later. Don't add lime at this point. | Conducting these experiments is fairly straight forward. Take the pre-treatment water and add salts to match the concentration of salts that you plan to your brewing water. In addition to these salts add some chalk to the water. This chalk will not dissolve but provides nucleation sites for the chalk that will precipitate from the water later. Don't add lime at this point. | ||
| − | Then divide this water | + | Then divide this water among 3 glass jars (Mason jars, beakers or small erlenmeyer flasks work well) giving each jar the same amount of water. Now calculate the amount of lime needed to precipitate all alkalinity in these small water samples. 85% of that amount is added to the first sample, 100% is added to the 2nd sample and 115% is added to the 3rd sample. Since the amounts added are rather small it is advantageous to prepare 5% by weight lime milk by simply mixing 10g lime with 90g water (tap water will work just fine). Once brought to an even suspension the amount lime can be measured with a pipette (1 ml = 0.05 g lime) or by weighing the solution (1g = 0.05g lime) |
| − | After the lime has been added mix the samples | + | After the lime has been added mix the samples thoroughly and let the precipitate settle over night. |
| − | Then test the clear water for alkalinity (KH) and, if possible, for pH. I did this for 5 different samples and the results are shown in Figure 3. As the amount of added lime is increased, the remaining alkalinity drops until a minimum is reached. After that no more additional alkalinity can be precipitated. The rise in alkalinity after that point stems from the sharp increase of | + | Then test the clear water for alkalinity (KH) and, if possible, for pH. I did this for 5 different samples and the results are shown in Figure 3. As the amount of added lime is increased, the remaining alkalinity drops until a minimum is reached. After that no more additional alkalinity can be precipitated. The rise in alkalinity after that point stems from the sharp increase of hydroxide ions (OH<sup>-</sup>) which is reflected by the rise in pH. Those residual hydroxide ions didn't find any bicarbonate ions or carbonic acid to react with. |
| − | The lime | + | The lime concentration that lead to the lowest alkalinity and an acceptable water pH (<9.5) should be used for the treatment of the water at hand. |
| valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| Line 203: | Line 201: | ||
| | | | ||
| − | = detailed calculation for | + | = detailed calculation for necessary calcium hydroxide additions = |
| − | This section is intended for brewers who want to incorporate the lime treatment option into their own spreadsheet. It details the calculations | + | This section is intended for brewers who want to incorporate the lime treatment option into their own spreadsheet. It details the calculations necessary and is thus only for brewers who want to know about that detail of the subject. |
| − | As mentioned earlier, to simplify the calculations we will base the necessary amount of lime on the amount of carbonic acid and bicarbonate that needs to be converted to carbonate. This neglects the amount | + | As mentioned earlier, to simplify the calculations we will base the necessary amount of lime on the amount of carbonic acid, carbon dioxide and bicarbonate that needs to be converted to carbonate. This neglects the amount needed to increase the OH<sup>-</sup> concentration which is a result of the rising pH. To illustrate the calculations I will be using the water profile from the spreadsheet demonstration. |
==calcium hydroxide needed== | ==calcium hydroxide needed== | ||
| − | To calculate the | + | To calculate the amount of calcium hydroxide needed the amounts of carbonic acid/carbon dioxide, bicarbonate and carbonate need to be calculated. This information can be determined from the alkalinity and the pH of the water. In its simplest definition, alkalinity is a measure of the bicarbonate and carbonate concentration in water. It is defined as the equivalent of strong acid it takes to convert virtually all bicarbonate and carbonate to carbonic acid or CO<sub>2</sub> by lowering the pH to 4.3 <ref name="deLange"/>. The following is an equation that works well for estimating the alkalinity A from the bicarbonate ([HCO<sub>3</sub><sup>-</sup>]) and carbonate concentrations ([CO<sub>3</sub><sup>2-</sup>]). |
{|style="width:800px" | {|style="width:800px" | ||
| Line 221: | Line 219: | ||
A chemical formula in brackets ([]) denotes the concentration of that substance in mmol/l. | A chemical formula in brackets ([]) denotes the concentration of that substance in mmol/l. | ||
| − | Now that we know the relationship between alkalinity and the bicarbonate and carbonate concentration we need a formula that expresses their relative concentration. For that we use the Henderson-Hasselbalch equation. Written for the | + | Now that we know the relationship between alkalinity and the bicarbonate and carbonate concentration we need a formula that expresses their relative concentration. For that we use the Henderson-Hasselbalch equation. Written for the ratio between bicarbonate and carbonate it looks like this |
{|style="width:800px" | {|style="width:800px" | ||
| Line 229: | Line 227: | ||
|} | |} | ||
| − | The | + | The second pKa for carbonic acid is 10.33 <ref name="wikipedia">[http://en.wikipedia.org/wiki/Carbonic_acid carbonic acid], Wikipedia.org</ref>. As explained in [[An_Overview_of_pH#Weak_acids_and_bases|Weak Acids and Bases]] the pKa is the pH at which the concentration of acid and conjugate base is equal. In our case the bicarbonate is the acid, since it sill has the proton (H<sup>+</sup>) and carbonate is the conjugate base. Rearranging (2) gives (3) which expresses the relative concentration of bicarbonate ([HCO<sub>3</sub><sup>-</sup>]) to carbonate ([CO<sub>3</sub><sup>2-</sup>]) |
{|style="width:800px" | {|style="width:800px" | ||
| Line 245: | Line 243: | ||
|} | |} | ||
| − | A bicarbonate concentration of 4.0848 mmol/l means that almost all of the 4.1 mEq/l | + | A bicarbonate concentration of 4.0848 mmol/l means that almost all of the 4.1 mEq/l alkalinity is present as bicarbonate which is to be expected for a pH of 7.6 which is fairly far away from the pK<sub>a2</sub> of 10.33. |
But the lime addition needs to convert more to carbonate than just the bicarbonate. It also needs to take care of the carbonic acid (H<sub>2</sub>CO<sub>3</sub>) and dissolved carbon dioxide (CO<sub>2</sub>). The combined concentration of the two can be found using (2) with the first pKa of carbonic acid (pK<sub>a1</sub> = 6.35, [Wikipedia]) which gives the following formula | But the lime addition needs to convert more to carbonate than just the bicarbonate. It also needs to take care of the carbonic acid (H<sub>2</sub>CO<sub>3</sub>) and dissolved carbon dioxide (CO<sub>2</sub>). The combined concentration of the two can be found using (2) with the first pKa of carbonic acid (pK<sub>a1</sub> = 6.35, [Wikipedia]) which gives the following formula | ||
| Line 255: | Line 253: | ||
|} | |} | ||
| − | While the formula is written with the concentration of carbonic acid (H<sub>2</sub>CO<sub>3</sub>) it technically refers to carbonic acid and carbon dioxide since the former is formed immediately from the pool of available CO<sub>2</sub> when some or all of it is converted to bicarbonate or carbonate. | + | While the formula is written with the concentration of carbonic acid (H<sub>2</sub>CO<sub>3</sub>) it technically refers to carbonic acid and carbon dioxide since the former is formed immediately from the pool of available CO<sub>2</sub> when some or all of it is converted to bicarbonate or carbonate<ref name="deLange_2">A.J. deLange, personal correspondence</ref>. |
Reordering (5) allows us to find the carbonic acid/CO<sub>2</sub> concentration from the bicarbonate concentration and the water pH | Reordering (5) allows us to find the carbonic acid/CO<sub>2</sub> concentration from the bicarbonate concentration and the water pH | ||
| Line 265: | Line 263: | ||
|} | |} | ||
| − | To precipitate the existing alkalinity as CaCO<sub>3</sub> both the bicarbonate and carbonic acid | + | To precipitate the existing alkalinity as CaCO<sub>3</sub> both the bicarbonate and carbonic acid need to be converted to carbonate. Each mole of bicarbonate takes one mole of hydroxide (OH<sub>-</sub>) and each mole of carbonic acid/CO<sub>2</sub> takes 2 moles of hydroxide. Therefore the following concentration of hydroxide needs to be added: |
{|style="width:800px" | {|style="width:800px" | ||
| Line 275: | Line 273: | ||
Each mole of calcium hydroxide (Ca(OH)<sub>2</sub>) adds 2 moles of hydroxide thus 4.5448 / 2 = 2.2724 mmol/l calcium hydroxide are needed. Calcium hydroxide has a molar mass of 74.1 g/mol and therefore its concentration by weight is 2.2724 * 74.1 = 168 mg/l (or ppm). | Each mole of calcium hydroxide (Ca(OH)<sub>2</sub>) adds 2 moles of hydroxide thus 4.5448 / 2 = 2.2724 mmol/l calcium hydroxide are needed. Calcium hydroxide has a molar mass of 74.1 g/mol and therefore its concentration by weight is 2.2724 * 74.1 = 168 mg/l (or ppm). | ||
| − | As mentioned earlier, these calculations have been simplified by assuming that all carbonic acid and bicarbonate will be converted to carbonate and that none of the added hydroxide ions are needed for the rise in pH which also requires a rise of the free OH<sup>-</sup> concentration. This is not exactly true but good enough for all intents and | + | As mentioned earlier, these calculations have been simplified by assuming that all carbonic acid and bicarbonate will be converted to carbonate and that none of the added hydroxide ions are needed for the rise in pH which also requires a rise of the free OH<sup>-</sup> concentration. This is not exactly true but good enough for all intents and purposes of this calculation. To rise the water pH of distilled water from 7 to 10, for example, it takes 3.7 mg/l of calcium hydroxide. |
==calcium balance== | ==calcium balance== | ||
| − | Once the amount of calcium hydroxide necessary to precipitate all alkalinity has been calculated, the calculation of the | + | Once the amount of calcium hydroxide necessary to precipitate all alkalinity has been calculated, the calculation of the calcium remaining after that precipitation is easy. In fact the amount of lime needed does not even play into it. All calcium added by the lime precipitates which becomes clear you look at the formulas given in [[#An unintuitive concept explained|An unintuitive concept explained]]. The amount of calcium that is actually removed from the water depends only on the amount of alkalinity that is precipitated. |
We also assume that all the pre-treatment water's alkalinity is present in the form of bicarbonates. If there were already a substantial amount of carbonate in the water, it would precipitate as calcium carbonate due to the presence of calcium. Each precipitating calcium ion removes 2 bicarbonate ions. One precipitates as carbonate with the calcium ion from the water and the other precipitates as calcium carbonate with the calcium ion from the added lime: | We also assume that all the pre-treatment water's alkalinity is present in the form of bicarbonates. If there were already a substantial amount of carbonate in the water, it would precipitate as calcium carbonate due to the presence of calcium. Each precipitating calcium ion removes 2 bicarbonate ions. One precipitates as carbonate with the calcium ion from the water and the other precipitates as calcium carbonate with the calcium ion from the added lime: | ||
| Line 289: | Line 287: | ||
|} | |} | ||
| − | For the aforementioned water profile with a calcium concentration of 76 mg/l (1.9 mmol/l) and an alkalinity of 205 ppm as CaCO<sub>3</sub> (4.1 mmpl/l bicarbonate) the calcium balance is negative. In practice it means that the alkalinity removal is limed by the calcium concentration (see [[#Limitations|Limitations]]. To fix that and | + | For the aforementioned water profile with a calcium concentration of 76 mg/l (1.9 mmol/l) and an alkalinity of 205 ppm as CaCO<sub>3</sub> (4.1 mmpl/l bicarbonate) the calcium balance is negative. In practice it means that the alkalinity removal is limed by the calcium concentration (see [[#Limitations|Limitations]]). To fix that and ensure that the post treatment water has sufficient calcium, gypsum and calcium chloride were added. This addition raises the calcium content to 102 mg/l (2.55 mmol/l) and the resulting calcium balance is positive: |
{|style="width:800px" | {|style="width:800px" | ||
| Line 305: | Line 303: | ||
= Reducing magnesium hardness = | = Reducing magnesium hardness = | ||
| − | So far only the reduction of alkalinity and calcium hardness though the precipitation of calcium carbonate has been discussed. However, water treatment with lime can also be used to reduce | + | So far only the reduction of alkalinity and calcium hardness though the precipitation of calcium carbonate has been discussed. However, water treatment with lime can also be used to reduce magnesium hardness. Elevated magnesium levels together with high sulfate levels lead to the formation of Epsom salt which can impart a bitter flavor. Epsom salt is even called ''Bittersalz'' (bitter salt) in German. |
| − | Magnesium carbonate is about 100 times more soluble than calcium carbonate | + | Magnesium carbonate is about 100 times more soluble than calcium carbonate which is why it doesn't precipitate as soon as the carbonate concentration is raised. Instead, magnesium needs to be precipitated as magnesium hydroxide which is by a factor of ~100 less soluble than calcium carbonate. The conversion from magnesium bicarbonate to magnesium hydroxide follows these two steps: |
:Mg<sup>2+</sup> + 2 HCO<sub>3</sub><sup>-</sup> + Ca<sup>2+</sup> + 2 OH<sup>-</sup> → Mg<sup>2+</sup> + CO<sub>3</sub><sup>2-</sup> + CaCO<sub>3</sub>↓ | :Mg<sup>2+</sup> + 2 HCO<sub>3</sub><sup>-</sup> + Ca<sup>2+</sup> + 2 OH<sup>-</sup> → Mg<sup>2+</sup> + CO<sub>3</sub><sup>2-</sup> + CaCO<sub>3</sub>↓ | ||
:Mg<sup>2+</sup> + CO<sub>3</sub><sup>2-</sup> + Ca<sup>2+</sup> + 2 OH<sup>-</sup> → Mg(OH)<sub>2</sub>↓ + CaCO<sub>3</sub>↓ | :Mg<sup>2+</sup> + CO<sub>3</sub><sup>2-</sup> + Ca<sup>2+</sup> + 2 OH<sup>-</sup> → Mg(OH)<sub>2</sub>↓ + CaCO<sub>3</sub>↓ | ||
| − | To precipitate one magnesium ion and two bicarbonate ions it takes two calcium hydroxide molecules. The calcium from the calcium hydroxide precipitates along with the magnesium hydroxide. | + | To precipitate one magnesium ion and two bicarbonate ions it takes two calcium hydroxide molecules. The calcium from the calcium hydroxide precipitates as calcium carbonate along with the magnesium hydroxide. |
| − | To make the | + | To make the aforementioned reactions happen the pH of the water needs to be raised to a pH of 10.5 - 11 <ref name="Narziss"/> which is well above the pH necessary for simple calcium carbonate precipitation. Furthermore the resulting water will have a pH that remains too high for use as brewing water. To solve this problem a 2 stage lime treatment is commonly used to precipitate magnesium. |
| − | The calcium hydroxide is added to about half of the pre treatment water until a pH of 10.5 - 11 is reached | + | The calcium hydroxide is added to about half of the pre-treatment water until a pH of 10.5 - 11 is reached. After reacting with the water the precipitate is allowed to settle. During this process both magnesium hydroxide and calcium carbonate are removed. The clear water is then removed from the sediment and mixed with the remaining amount of pre treatment water which results in an additional precipitation of calcium carbonate but no magnesium hydroxide or magnesium carbonate. The post-treatment water pH from this 2nd stage will fall far enough to create suitable brewing water. |
| − | Such 2 stage water treatment is able to reduce the magnesium content of the water by 50-65% | + | Such 2 stage water treatment is able to reduce the magnesium content of the water by 50-65% <ref name="Narziss"/> |
| valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| Line 328: | Line 326: | ||
[[Image:Lime_single_stage_water_treatment.gif|frame|right|'''Figure 11''' - Schematic drawing of simple single stage alkalinity reduction system. See text for explanation.]] | [[Image:Lime_single_stage_water_treatment.gif|frame|right|'''Figure 11''' - Schematic drawing of simple single stage alkalinity reduction system. See text for explanation.]] | ||
| − | Many breweries perform | + | Many breweries perform the water treatment discussed in this article with continuously operating systems. Figure 1 depicts a simple 1 stage system that can be used to precipitate calcium carbonate from an incoming stream of "raw"-water. |
| − | + | A part of the incoming water is diverted into a calcium hydroxide holding tank where it saturates with calcium hydroxide. How much depends on the alkalinity to be precipitates since it controls the amount of calcium hydroxide that will be mixed with the water. The main water flow and the calcium hydroxide saturated water are thoroughly mixed in a reactor. As the mixed water flows down a center pipe calcium carbonate precipitates. Once the water reaches the bottom it flows upwards where the available cross section is much larger and the resulting flow is slower. So slow in fact that the precipitating calcium carbonate is able to settle and the water exiting at the top of the reactor is almost clear. The following gravel filter removes residual haze from the water and the water leaving the treatment system is clear and of lower alkalinity and hardness than the pre-treatment water. As part of maintenance of the system calcium carbonate has to be removed from the precipitate and calcium hydroxide has to be added to its holding tank [Narziss&Back, 2009] | |
| − | Modern modifications to this system use | + | Modern modifications to this system use Calcium carbonate granules or silica sand (0.2 - 0.5 mm grain size) in the reactor which provide crystallization sites for calcium carbonate and shorten the time spent in the reactor to 60-90 min. Multi-stage units, which are able to precipitate magnesium as well, are also available <ref name="Narziss"/>. |
| valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| Line 340: | Line 338: | ||
= Final remarks = | = Final remarks = | ||
| − | This particular water treatment method is very popular among large brewers who need to deal with high water hardness and | + | This particular water treatment method is very popular among large brewers who need to deal with high water hardness and alkalinity in a cost effective way but it is rarely used among home brewers. I think the reason for that to some extend clear: it is more complicated and less intuitive than treating water with acids, diluting it or even building brewing water from scratch. However, it certainly is a process that can be made to work by the advanced home brewer and once established may become standard procedure when brewing lighter beer styles which require softer water. Even if it doesn't become standard procedure some brewers may want to give it a try just to say: ''been there, done that''. |
=References= | =References= | ||
| − | + | <references/> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|} | |} | ||
Latest revision as of 02:16, 1 April 2010
|
High alkalinity in their brewing water keeps many home brewers from being able to brew lighter beers well. To overcome this obstacle many of them use reverse osmosis water to dilute their water or even build their brewing water from scratch through the addition of salts. Another option is the use of acids to neutralize some or all of the water's alkalinity. If most or all of the water's alkalinity is the result of temporary hardness (dissolved calcium and magnesium carbonate) another options exists which is commonly overlooked: alkalinity precipitation using calcium hydroxide (also known as slaked lime). Due to its low cost this method of water treatment is widely used in the brewing industry but home brewers appear to have largely stayed away from it due a lack of understanding of the chemistry behind it and missing support in brewing water calculators. Based on A.J. deLange's work [1] and through pointers in technical brewing literature [2] I was able to get it to work with my water and add support for the necessary calculations to the water calculation spreadsheet (Kaiser_water_calculator.xls). The following article attempts to shed light on this process and provide a better understanding of the chemistry behind it which hopefully allows other brewers to use this elegant form of water treatment in their brewing process. Contents
An unintuitive concept explained | |||||||||||||||||||
|
Most brewing books mention that the alkalinity and hardness of brewing water can be lowered through the addition of a lime (calcium hydroxide) which itself is a strong alkali. How can the addition of a strongly alkaline compound like slaked like reduce alkalinity? This always confused me and it took me a while until the chemistry behind this process became clear. To better understand the chemical process described here the reader should have read An Overview of pH as well as About chalk and the carbonate system. Calcium hydroxide is a strong base and as such it dissociates into calcium and hydroxide regardless of the water pH when it dissolves in water:
The resulting hydroxide ions (OH-) want to raise the pH of the solution and in pure water will be able to that very effectively. However, the water we are treating with lime contains bicarbonate and carbonic acid with acts as a pH buffer and by doing so stand in the way of a large pH rise. While we have experienced alkalinity only as a hindrance for lowering the pH of the mash a pH buffer works both ways. I.e. it keeps the pH from falling as much as as it keeps it from rising. 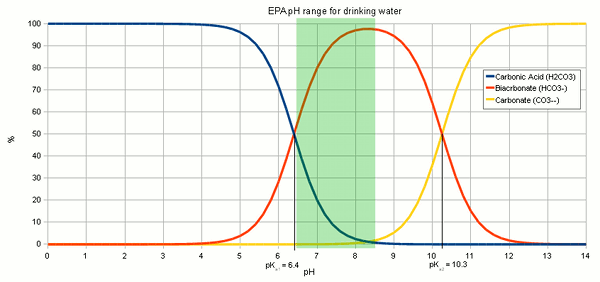 Figure 1 - The relative concentration of carbonic acid, bicarbonate and carbonate based on the pH of the solution. The EPA (Environmental Protection Agency) recommends drinking water to have a pH between 6.5 and 8.5 [3] Since the ratio of carbonic acid, bicarbonate and carbonate stands in close relation to the pH of the solution (Figure 1) the hydroxide ions from the lime need to react with the carbonic acid (H2CO3), carbon dioxide (CO2 that hasn't formed carbonic acid yet) and bicarbonate (HCO-3) to increase the concentration of carbonate (CO2-3) through the following reactions
The added hydroxide is basically sucking the hydrogen ions (H+) from both carbonic acid and bicarbonate to covert them to carbonate. This corresponds to traversing to the right in Figure 2. At some point most of the hydroxide ions have been consumed by aforementioned reactions and the carbonic acid/bicarbonate/carbonate balance is such that it is biased heavily towards carbonate. This happened with a rise in pH since the relationship between pH and carbonic acid/bicarbonate/carbonate, which is shown in Figure 1, has to remain satisfied. But what matters for us is not the risen pH but the increased concentration of carbonate ions. At this point we have to look at the solubility equation for calcium carbonate which has already been mentioned in About chalk and the carbonate system:
If the product between the concentration of calcium and carbonate ions is greater than 3.36×10-9, which is a very small number, calcium carbonate will precipitate out of solution until either the calcium concentration or the carbonate concentration is low enough to satisfy this equation. When this precipitation happens a milky white haze comprised of calcium carbonate (chalk) forms which slowly settles.
The down arrow (↓) means that the formed chalk is precipitating. This precipitating chalk represents the temporary hardness of the water and the clear water that remains has a lower alkalinity and a lower hardness (i.e lower calcium content) than before. In short: adding lime raises the pH and pushes calcium and bicarbonate out of solution as calcium carbonate. |
| ||||||||||||||||||
LimitationsThis form of water treatment can only reduce alkalinity if sufficient calcium is present since it relies on the formation of calcium carbonate. Though changes can be made to the procedure (see Reducing magnesium hardness) the amount of magnesium that can be removed with this method is limited due to the fact that magnesium carbonate is about 100 times more soluble than calcium carbonate[4]. Sodium cannot be reduced with this method since sodium carbonate is very soluble in water. What cannot be reduced either are sulfate and chloride anions. Those will remain unchanged unless more are added though the addition of calcium salts, a practice that is commonly used to boost the water's calcium content and ensure an adequate calcium concentration in the treated water. Since alkalinity is only precipitated to the same extend that calcium is precipitated it is important that the calcium hardness exceeds the alkalinity. Calcium hardness is just another way of expressing the calcium content of the water. Rather then using the weight of the calcium, which is done when using ppm or mg/l as unit, the calcium content is represented as an equivalent amount of CaCO3 for example. To convert the calcium content from mg/l or ppm to ppm as CaCO3 simply multiply the ppm value with 2.5. Example: The water contains 100 ppm calcium and has an alkalinity of 200 ppm as CaCO3. How much calcium remains after all alkalinity has been precipitated? The calcium hardness of the water is 250 ppm as CaCO3. Removing 200 ppm worth of alkalinity in the form of chalk also requires that the calcium hardness is reduced by 200 ppm. The remaining calcium hardness is 50 ppm as CaCO3 or 20 mg/l. Because of the precipitation of calcium it might be necessary to supplement the water with calcium using calcium carbonate or gypsum. This is best done before or together with the lime addition. The amount needed depends on the expected chalk precipitation and the targeted calcium level of the post-treatment water. All of the calcium, which is added by the lime, will also be precipitated in the form of calcium carbonate. A fact that provides compliance with the German Reinheitsgebot since in the end nothing is added to the water [2]. In practice this method is not able to completely reduce the alkalinity to 0 regardless of the amount of calcium that is present. The practical lower limit of alkalinity reduction is 30-50 ppm as CaCO3. One reason for that is absorption of atmospheric CO2 during the settling time which causes some of the precipitating chalk to be dissolved again. While the reaction also takes place at low water temperatures the precipitating calcium carbonate forms larger particles in slightly warmer water and as a result the temperature of the treated water should not be below 12 C (54 F) [2] |
| ||||||||||||||||||
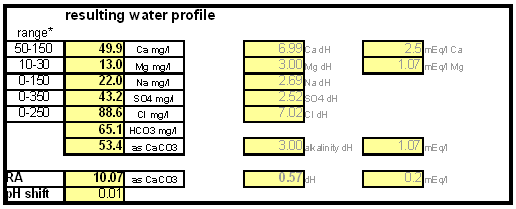
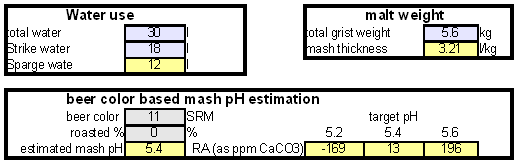
How to use the spreadsheetTo make water treatment with lime work the necessary amount of lime needs to be calculated. For the interested brewer the detailed calculations are explained later but I expect that many will take the easier route and use the existing water treatment spread sheet (Kaiser_water_calculator.xls). The pre-treatment waterAll calculations for lime additions are done on the "advanced" sheet of the spreadsheet. The first step is entering the water profile of the starting water. This can be taken from a full analysis or a GH&KH water test. In my case I have a full analysis of my well water which happens to be very similar to Munich water. It has a high level of temporary hardness, something that shows itself though the precipitation of chalk whenever this water is boiled. Lime needed and calcium balanceNow jump ahead to the "water treatment with lime" section. Here the current water pH and the water amount to be treated need to be specified. The water pH is used to determine how much carbonic acid and CO2 are present in the water. This is necessary since the added lime also needs to convert that to carbonate in addition to converting the bicarbonate to carbonate. The water amount to be treated is different from the total brewing water amount specified later since some water is left behind to separate the precipitate from the treated water. The result of the calculation is the theoretical lime amount, as ppm or mg/l, that needs to be added to precipitate all the water's alkalinity. But the more important result that needs attention here is the calcium surplus. This is the amount of calcium that would remain in the water if all alkalinity is precipitated with this process. If that concentration is below 10 ppm you need to add some calcium salts (Gypsum or Calcium Chloride) to boost the calcium content of the water. In my case I had to do that which will be explained in the next section. There are two more fields that require values: GH and KH. These are measurements from a GH&KH water test test which are taken from the treated water after calcium carbonate has precipitated and settled. Since the alkalinity reduction is rarely 100 % effective, testing the post treatment water and using this information along with information from the pre-treatment water is a more reliable approach than assuming an arbitrary efficiency for the lime treatment. For determining the final water profile it is assumed that lime treatment only precipitated alkalinity (i.e. bicarbonates and carbonates) and calcium. All other ions remain unaffected. Addition of saltsJumping back to the "water modification using salts" section. As mentioned earlier, the water used here contains more alkalinity than calcium hardness which limits the efficiency of the alkalinity removal and causes a calcium deficiency in the final water. To avoid this I decided to boost the water's calcium level with 20 ppm gypsum and 80 ppm calcium chloride. By doing so the calcium, sulfate and chloride levels of the water were raised. Their new concentrations in the pre-treatment water can be seen on the right most column. Salt and lime amounts needs for water treatmentWhen treating water with lime the necessary salts and lime are added to a water amount that is larger than what is needed for brewing. That's why there is a separate section labeled "necessary salt additions for lime treatment" which calculates the salt amounts based on the salt and lime concentrations needed well as the water amount specified earlier. It is unlikely that baking soda is added to the water when alkalinity removal is the goal. Though chalk will be added as well it is only added as a process aid and will not be carried over into the post-treatment water. This is why the list of salts is limited to gypsum, Epsom salt, table salt, calcium chloride and magnesium chloride. The amount of lime needed is given both as the amount of the dry power or a 5% (by weight) solution. The latter is useful for the treatment of small water amounts for test batches which will be explained later. Resulting water profileOnce GH and KH values for the post-treatment water have been entered in the "water treatment with lime" section, the resulting water profile is available. You'll notice that only calcium and bicarbonate/alkalinity changed (except for ions added by additional salts). As mentioned before this a result of the assumption that only calcium carbonate was precipitated which is valid since the precipitation of magnesium is more difficult. In this example lime treatment lowered the residual alkalinity from 143 ppm as CaCO3 to 10 ppm as CaCO3 Mash pH estimationAfter strike water, grist weight and beer color have been specified the mash pH can be estimated. In my case a 11 SRM Maibock was brewed and the estimated mash pH of 5.4 was close enough to the actual mash pH of 5.5. The pH estimation is less accurate when most of the color is coming from base malts which was the case for that grist What is neededTo efficiently treat water with lime you'll need a few supplies, some of which you may already have available. Food grade calcium hydroxide, a.k.a. slaked lime or hydrated lime, can be found in the canning section of many grocery stores under the name pickling lime. If your local stores don't carry it you can always order it from on-line merchants. A 1lb bag will last a long time.
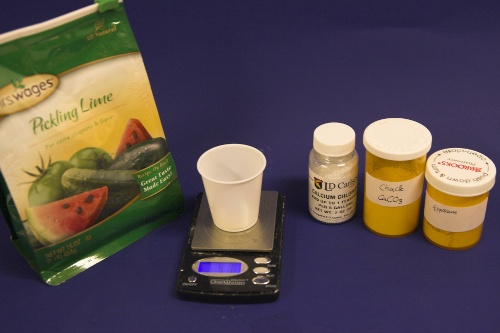
Though technically not necessary, a GH&KH water test kit is a very useful tool which allows you to check the results of the water treatment instantly. Without a means of measuring alkalinity you'll have to wait until you can measure the mash pH to confirm that the water treatment was successful. A large vessel for treating the water and letting the precipitate settle is also needed. To measure the salt and lime amounts a scale with a resolution of 0.1 g or better is needed. |
| ||||||||||||||||||
How to treat brewing water with limeIf you are planing to use this technique in your brewery on a regular basis here are some tips:
|
| ||||||||||||||||||
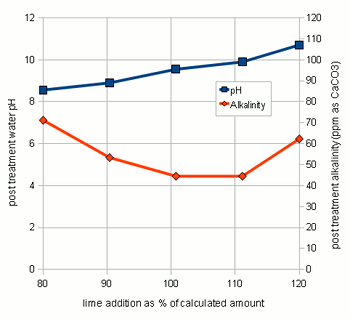
experimenting with the amount of lime actually neededThrough experiments I found that the amount of lime calculated by the spread sheet is not necessarily the optimal amount that gives the best alkalinity reduction. This might be the result of inaccuracies in the water analysis data and simplifications that were made for the calculations. As a result it is recommended to test the lime amounts in a small series of experiments where varying amounts of lime are added to the water and the alkalinity of the resulting water is tested. Conducting these experiments is fairly straight forward. Take the pre-treatment water and add salts to match the concentration of salts that you plan to your brewing water. In addition to these salts add some chalk to the water. This chalk will not dissolve but provides nucleation sites for the chalk that will precipitate from the water later. Don't add lime at this point. Then divide this water among 3 glass jars (Mason jars, beakers or small erlenmeyer flasks work well) giving each jar the same amount of water. Now calculate the amount of lime needed to precipitate all alkalinity in these small water samples. 85% of that amount is added to the first sample, 100% is added to the 2nd sample and 115% is added to the 3rd sample. Since the amounts added are rather small it is advantageous to prepare 5% by weight lime milk by simply mixing 10g lime with 90g water (tap water will work just fine). Once brought to an even suspension the amount lime can be measured with a pipette (1 ml = 0.05 g lime) or by weighing the solution (1g = 0.05g lime) After the lime has been added mix the samples thoroughly and let the precipitate settle over night. Then test the clear water for alkalinity (KH) and, if possible, for pH. I did this for 5 different samples and the results are shown in Figure 3. As the amount of added lime is increased, the remaining alkalinity drops until a minimum is reached. After that no more additional alkalinity can be precipitated. The rise in alkalinity after that point stems from the sharp increase of hydroxide ions (OH-) which is reflected by the rise in pH. Those residual hydroxide ions didn't find any bicarbonate ions or carbonic acid to react with. The lime concentration that lead to the lowest alkalinity and an acceptable water pH (<9.5) should be used for the treatment of the water at hand. |
| ||||||||||||||||||
detailed calculation for necessary calcium hydroxide additionsThis section is intended for brewers who want to incorporate the lime treatment option into their own spreadsheet. It details the calculations necessary and is thus only for brewers who want to know about that detail of the subject. As mentioned earlier, to simplify the calculations we will base the necessary amount of lime on the amount of carbonic acid, carbon dioxide and bicarbonate that needs to be converted to carbonate. This neglects the amount needed to increase the OH- concentration which is a result of the rising pH. To illustrate the calculations I will be using the water profile from the spreadsheet demonstration. calcium hydroxide neededTo calculate the amount of calcium hydroxide needed the amounts of carbonic acid/carbon dioxide, bicarbonate and carbonate need to be calculated. This information can be determined from the alkalinity and the pH of the water. In its simplest definition, alkalinity is a measure of the bicarbonate and carbonate concentration in water. It is defined as the equivalent of strong acid it takes to convert virtually all bicarbonate and carbonate to carbonic acid or CO2 by lowering the pH to 4.3 [1]. The following is an equation that works well for estimating the alkalinity A from the bicarbonate ([HCO3-]) and carbonate concentrations ([CO32-]).
A chemical formula in brackets ([]) denotes the concentration of that substance in mmol/l. Now that we know the relationship between alkalinity and the bicarbonate and carbonate concentration we need a formula that expresses their relative concentration. For that we use the Henderson-Hasselbalch equation. Written for the ratio between bicarbonate and carbonate it looks like this
The second pKa for carbonic acid is 10.33 [5]. As explained in Weak Acids and Bases the pKa is the pH at which the concentration of acid and conjugate base is equal. In our case the bicarbonate is the acid, since it sill has the proton (H+) and carbonate is the conjugate base. Rearranging (2) gives (3) which expresses the relative concentration of bicarbonate ([HCO3-]) to carbonate ([CO32-])
Using (1) and (3) we can solve for the bicarbonate concentration which depends on both the alkalinity and the pH of the water. In our example the alkalinity is 205 ppm as CaCO3. To convert this to mEq/l, which is the unit needed by (1) simply divide by 50. This division by 50 stems from the molecular weight of calcium carbonate (100 g/mol) and the fact that each mol of calcium carbonate can neutralize 2 acid equivalents.
A bicarbonate concentration of 4.0848 mmol/l means that almost all of the 4.1 mEq/l alkalinity is present as bicarbonate which is to be expected for a pH of 7.6 which is fairly far away from the pKa2 of 10.33. But the lime addition needs to convert more to carbonate than just the bicarbonate. It also needs to take care of the carbonic acid (H2CO3) and dissolved carbon dioxide (CO2). The combined concentration of the two can be found using (2) with the first pKa of carbonic acid (pKa1 = 6.35, [Wikipedia]) which gives the following formula
While the formula is written with the concentration of carbonic acid (H2CO3) it technically refers to carbonic acid and carbon dioxide since the former is formed immediately from the pool of available CO2 when some or all of it is converted to bicarbonate or carbonate[6]. Reordering (5) allows us to find the carbonic acid/CO2 concentration from the bicarbonate concentration and the water pH
To precipitate the existing alkalinity as CaCO3 both the bicarbonate and carbonic acid need to be converted to carbonate. Each mole of bicarbonate takes one mole of hydroxide (OH-) and each mole of carbonic acid/CO2 takes 2 moles of hydroxide. Therefore the following concentration of hydroxide needs to be added:
Each mole of calcium hydroxide (Ca(OH)2) adds 2 moles of hydroxide thus 4.5448 / 2 = 2.2724 mmol/l calcium hydroxide are needed. Calcium hydroxide has a molar mass of 74.1 g/mol and therefore its concentration by weight is 2.2724 * 74.1 = 168 mg/l (or ppm). As mentioned earlier, these calculations have been simplified by assuming that all carbonic acid and bicarbonate will be converted to carbonate and that none of the added hydroxide ions are needed for the rise in pH which also requires a rise of the free OH- concentration. This is not exactly true but good enough for all intents and purposes of this calculation. To rise the water pH of distilled water from 7 to 10, for example, it takes 3.7 mg/l of calcium hydroxide. calcium balanceOnce the amount of calcium hydroxide necessary to precipitate all alkalinity has been calculated, the calculation of the calcium remaining after that precipitation is easy. In fact the amount of lime needed does not even play into it. All calcium added by the lime precipitates which becomes clear you look at the formulas given in An unintuitive concept explained. The amount of calcium that is actually removed from the water depends only on the amount of alkalinity that is precipitated. We also assume that all the pre-treatment water's alkalinity is present in the form of bicarbonates. If there were already a substantial amount of carbonate in the water, it would precipitate as calcium carbonate due to the presence of calcium. Each precipitating calcium ion removes 2 bicarbonate ions. One precipitates as carbonate with the calcium ion from the water and the other precipitates as calcium carbonate with the calcium ion from the added lime:
For the aforementioned water profile with a calcium concentration of 76 mg/l (1.9 mmol/l) and an alkalinity of 205 ppm as CaCO3 (4.1 mmpl/l bicarbonate) the calcium balance is negative. In practice it means that the alkalinity removal is limed by the calcium concentration (see Limitations). To fix that and ensure that the post treatment water has sufficient calcium, gypsum and calcium chloride were added. This addition raises the calcium content to 102 mg/l (2.55 mmol/l) and the resulting calcium balance is positive:
Calcium has an atomic weight of about 40 g/mol and thus the theoretical calcium content after all alkalinity has been precipitated is 0.5 * 40 = 20 mg/l |
| ||||||||||||||||||
Reducing magnesium hardnessSo far only the reduction of alkalinity and calcium hardness though the precipitation of calcium carbonate has been discussed. However, water treatment with lime can also be used to reduce magnesium hardness. Elevated magnesium levels together with high sulfate levels lead to the formation of Epsom salt which can impart a bitter flavor. Epsom salt is even called Bittersalz (bitter salt) in German. Magnesium carbonate is about 100 times more soluble than calcium carbonate which is why it doesn't precipitate as soon as the carbonate concentration is raised. Instead, magnesium needs to be precipitated as magnesium hydroxide which is by a factor of ~100 less soluble than calcium carbonate. The conversion from magnesium bicarbonate to magnesium hydroxide follows these two steps:
To precipitate one magnesium ion and two bicarbonate ions it takes two calcium hydroxide molecules. The calcium from the calcium hydroxide precipitates as calcium carbonate along with the magnesium hydroxide. To make the aforementioned reactions happen the pH of the water needs to be raised to a pH of 10.5 - 11 [2] which is well above the pH necessary for simple calcium carbonate precipitation. Furthermore the resulting water will have a pH that remains too high for use as brewing water. To solve this problem a 2 stage lime treatment is commonly used to precipitate magnesium. The calcium hydroxide is added to about half of the pre-treatment water until a pH of 10.5 - 11 is reached. After reacting with the water the precipitate is allowed to settle. During this process both magnesium hydroxide and calcium carbonate are removed. The clear water is then removed from the sediment and mixed with the remaining amount of pre treatment water which results in an additional precipitation of calcium carbonate but no magnesium hydroxide or magnesium carbonate. The post-treatment water pH from this 2nd stage will fall far enough to create suitable brewing water. Such 2 stage water treatment is able to reduce the magnesium content of the water by 50-65% [2] |
| ||||||||||||||||||
Water treatment in a large breweryMany breweries perform the water treatment discussed in this article with continuously operating systems. Figure 1 depicts a simple 1 stage system that can be used to precipitate calcium carbonate from an incoming stream of "raw"-water. A part of the incoming water is diverted into a calcium hydroxide holding tank where it saturates with calcium hydroxide. How much depends on the alkalinity to be precipitates since it controls the amount of calcium hydroxide that will be mixed with the water. The main water flow and the calcium hydroxide saturated water are thoroughly mixed in a reactor. As the mixed water flows down a center pipe calcium carbonate precipitates. Once the water reaches the bottom it flows upwards where the available cross section is much larger and the resulting flow is slower. So slow in fact that the precipitating calcium carbonate is able to settle and the water exiting at the top of the reactor is almost clear. The following gravel filter removes residual haze from the water and the water leaving the treatment system is clear and of lower alkalinity and hardness than the pre-treatment water. As part of maintenance of the system calcium carbonate has to be removed from the precipitate and calcium hydroxide has to be added to its holding tank [Narziss&Back, 2009] Modern modifications to this system use Calcium carbonate granules or silica sand (0.2 - 0.5 mm grain size) in the reactor which provide crystallization sites for calcium carbonate and shorten the time spent in the reactor to 60-90 min. Multi-stage units, which are able to precipitate magnesium as well, are also available [2]. |
| ||||||||||||||||||
Final remarksThis particular water treatment method is very popular among large brewers who need to deal with high water hardness and alkalinity in a cost effective way but it is rarely used among home brewers. I think the reason for that to some extend clear: it is more complicated and less intuitive than treating water with acids, diluting it or even building brewing water from scratch. However, it certainly is a process that can be made to work by the advanced home brewer and once established may become standard procedure when brewing lighter beer styles which require softer water. Even if it doesn't become standard procedure some brewers may want to give it a try just to say: been there, done that. References
|