Difference between revisions of "Sandbox"
| Line 239: | Line 239: | ||
While the malt adds potassium on the order of 500 mg/l to the mash the potassium content of the brewing water should be less than 10 mg/l <ref name="Narziss"/>. | While the malt adds potassium on the order of 500 mg/l to the mash the potassium content of the brewing water should be less than 10 mg/l <ref name="Narziss"/>. | ||
| + | |||
| + | === other metal ions === | ||
| + | |||
| + | Iron causes problems at concentrations as low as 0.2 mg/l by inhibiting sacharification, increasing color of the wort and promote beer staling processes <ref name="Narziss"/>. Excessive iron can also give the beer a metallic blood-like taste. Iron is part of the secondary (i.e. cosmetic) water standards and its level is recommended to be below 0.3 mg/l <ref name=”EPA SMCL”>EPA, [http://www.epa.gov/safewater/consumer/2ndstandards.html Secondary Drinking Water Regulations]</ref> | ||
| + | |||
| + | Manganese is important for enzymes in yeast metabolism but excessive amounts cause the same problems as excessive iron content. The EPA recommends a manganese concentration of 0.05 mg/l or lower <ref name=”EPA SMCL”/>. | ||
| + | |||
| + | Many other metals like copper, zinc, lead and tin are toxic to yeast when present in higher concentrations but important for yeast metabolism and growth at lower concentrations <ref name="Narziss"/>. However, for successful fermentation none of these metals have to come from the brewing water. In the case of beer the malt is providing an adequate amount of these trace elements. | ||
| + | |||
| + | === bicarbonate/carbonate (HCO<sub>3</sub><sup>-</sup>/ CO<sub>3</sub><sup>2-</sup>=== | ||
| + | |||
| + | Molecular weight: 61.01 g/mol (HCO<sub>3</sub><sup>-</sup>); 60.01 g/mol (CO<sub>3</sub><sup>2-</sup>) | ||
| + | |||
| + | Bicarbonate and carbonate, which are the [#pH overview|conjugate bases] of carbonic acid, are responsible for the water’s pH buffering capacity which is expressed in its alkalinity. As a result of that they can be used to predict the water’s alkalinity. Other ions that have pH buffering properties are phosphates which are generally not present in significant amounts. | ||
| + | |||
| + | The increased pH buffering brought on by bicarbonate and carbonate works against the acidity of the grist and can lead high mash and boil pH values. The effect of high mash and boil pH has been discussed in [#pH in brewing] | ||
| + | |||
| + | If the water also contains significant amounts of calcium, carbonate is generally not present in any significant amount since it forms a poorly soluble salt wit calcium: calcium carbonate or chalk which eventually precipitates from the water. As a result the water pH tends to be below 9 at which point bicarbonate is the dominant ion that represents the water’s alkalinity. | ||
| + | |||
| + | === Sulfate === | ||
| + | |||
| + | The sulfate ion (SO<sub>4</sub><sup>2-</sup>) affects the perception of bitterness. High sulfate levels give the beer a dry and more bitter taste [Narziss&Back]. | ||
| + | |||
| + | === Chloride === | ||
| + | |||
| + | The chloride ion is known to enhance the activity of the alpha-amylase [Narziss&Back], <ref name="Eyster"> C. Eyester, [https://kb.osu.edu/dspace/bitstream/1811/4633/1/V59N05_257.pdf The Optimum pH For Diastase Of Malt Activity], 1959, The Ohio Journal Of Science</ref> by binding to the enzyme and transforming it into a more active state <ref name=”Hengge”> A. Hengge, Faculty, Chemistry and Biochemistry, Utah State University, [http://www.madsci.org/posts/archives/2000-04/956616009.Bc.r.html post on www.madsci.org</ref>. | ||
| + | |||
| + | Used as calcium chloride it gives the beer a full and smooth taste. Sodium chloride enhances the beer’s mouthfeel [Narziss&Back]. | ||
| + | |||
| + | Chloride dominated waters tend to be better for malt dominated beers while waters high in sulfate are more suitable for hoppy and more bitter beers. | ||
|} | |} | ||
Revision as of 01:23, 18 May 2010
| |
Brewing Basics: The building blocks stand for basic stuff that is important for the understanding of further discussions and elaborations. |
| |
How Things Work: the cogs mark sections that detail how a particular process woks |
| |
Practical Brewing Advice: The pot stands for practical brewing advice that will help you in home brewing. Oftentimes a conclusion that is drawn from preceding, more complex, content. |
| |
Geeky Stuff: The test tube stands for geeky content. Something that is cool to know but only has little importance in practical home brewing. |
this is a reference with html [2] and another one [3]
| × | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 1 | 2 | 3 |
| 2 | 2 | 4 | 6 |
| 3 | 3 | 6 | 9 |
| 4 | 4 | 8 | 12 |
| 5 | 5 | 10 | 15 |
Contents
References
- ↑ test 1
- ↑ [1]
- ↑ A.J. deLange, Alkalinity, Hardness, Residual Alkalinity and Malt Phosphate: Factors in the Establishment of Mash pH, 2004,
|
There is a point in many home brewers’ career when they become curious about the main ingredient: water. In many cases the water coming from the tap is adequate for brewing, which is why home brewers rarely worry about their water for their first batches. When starting out getting comfortable with the brewing process is more important than having to worry about water chemistry, which can get quite complex. But once there is a need or simply the curiosity to know what is in the water and how it can affect the beer a water analysis is needed. This article focuses how to obtain a water analysis and, more importantly, how to read and interpret the data that is given in it.
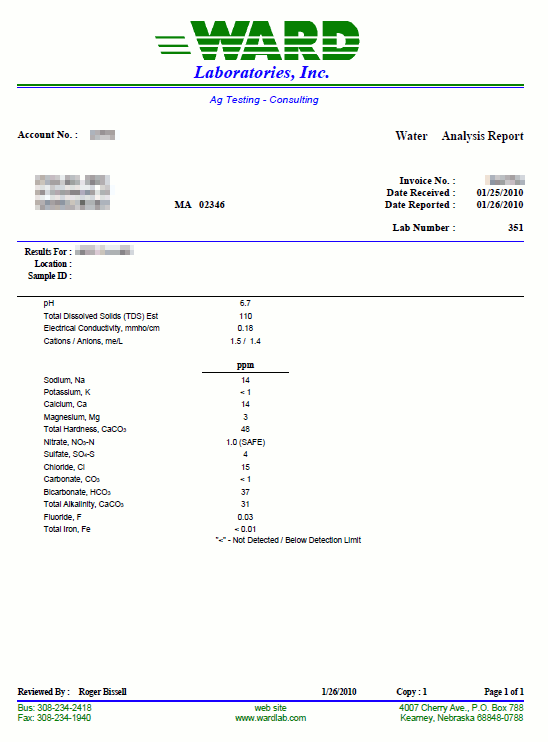
Obtaining a water reportThere are a number of sources for a water report. If you are on municipal water the first stop would be the website of your water department. Unfortunately, most municipalities post only a water quality report which not necessarily lists all the minerals that were brewers are interested in. The reason for that is that those minerals don't pose a health risk and their actual concentration has little effect on the water's suitability as drinking water. However, most of the water quality reports that I have seen list alkalinity and total hardness which can tell a lot about the water's effect on the mash and boil pH. In the US, the EPA (Environmental Protection Agency) requires that drinking water suppliers provide their customers with a Consumer Confidence Report on a yearly basis[1]. For brewing, a water report should list these minerals and parameters:
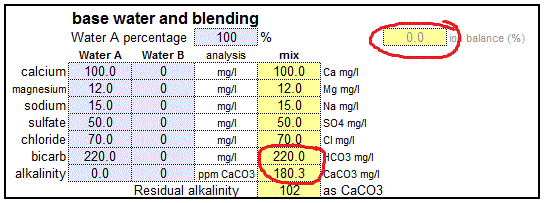
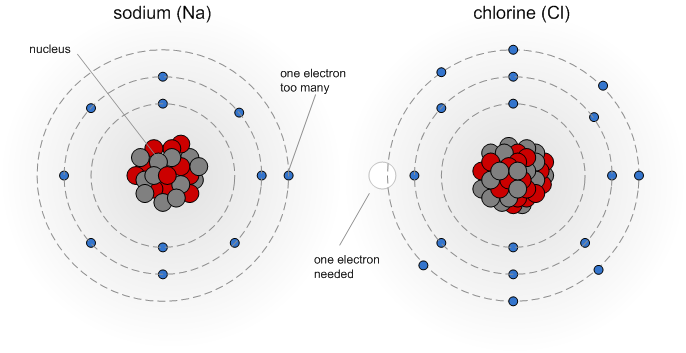
If your water department doesn’t provide that information or you get your water from a private well, there are a few alternatives. One option is to call the water department and ask if they can provide the information. However, many brewers opt for sending their water to a lab for a custom water analysis. One very popular water lab among home brewers is Ward Laboratories Inc. (wardlab.com) in Kearney Nebraska. The water analysis to get is W-6 Household Mineral Test which costs $16.50. Simply clean and fill a ½ pint or pint sized plastic bottle with the water to be tested. Send that water with a check for the analysis fee and a note which indicates: you want analysis W-6, your mailing address and your e-mail address to the address given here. A few days later you'll receive your water report as an e-mail. It's that simple. An even quicker option, which works well if you are only interested in a crude analysis is a GH & KH water test. Those tests are designed to be done at home. It is also a very practical means to detect changes in your water which may require a new water analysis to be done. As I will show later, total hardness (GH) and alkalinity (KH) are sufficient to make a reasonable accurate prediction of the mash pH or necessary water treatments to correct the pH of the mash. Atoms, molecules and ionsThe minerals, which are dissolved in water are present there as ions. To better understand what atoms, ions and molecules are let’s take a closer look at basic chemistry. Don’t worry; I won’t go to deep into it. Atoms are the basic building blocks of chemical compounds (Figure 2). They consist of a nucleus which is build from positively charged protons and neutral neutrons. It represents the vast majority of the atom’s weight. The nucleus is surrounded by negatively charged electrons. In an atom there is one electron for each proton and as a result the overall electrical charge of the atom is neutral. It is the number and configuration of these electrons that determine the chemical behavior of an atom. Let’s look at the example of sodium and chlorine below Sodium has 11 electrons. A simple model of their configuration is shown in Figure 2. The shell closest to the nucleus can hold 2 electrons and is completely filled. The next shell can hold 8 electrons and is completely filled too. The outermost shell, however, can also hold 8 electrons but holds only one electron. It is a basic law of physics that an atom always strives to having fully completed electron shells. This causes most of them (except the noble gases which naturally have these configuration) to reach with other atoms as we will see shortly. The chlorine atom has the opposite problem; it is missing one electron to complete its outer shell of electrons. So, when they both get together the sodium donates one of its electrons to the chlorine. By doing so the sodium has 2 complete electron shells and the chloride has 3 complete shells. Both atoms are now “happy”, but this electron transfer left them electrically charged. The sodium, now a sodium ion, lost one electron and has one positive charge. The chlorine, now a chloride ion, has gained one electron and therefore has one negative charge. [Image: Sodiumchloride.png|frame|center|Figure 3 – The now charged sodium and chloride ions are attracted to each other and are forming a sodium chloride molecule] Two oppositely charges ions attract each other and also arrange themselves in a regular structure just like a Magnetix toy. The results are the salt crystals we all know. But that’s beside the point. Important is what happens to sodium chloride when it comes in contact with water. Because of the arrangement of the hydrogen atoms the water molecule has a positively and a negatively charged side. This property of water, being a polar liquid, makes it a great solved for anything charged. Just like the sodium and chloride ions in sodium cloride. [Image: Sodium_and_chloride_in_water.png|frame|center|Figure 4 – sodium chloride dissolved in water] Because of their polar nature those water molecules are able to get between the sodium and chloride ions and break up their bond. The salt dissolves. Based on the number of electrical charges, ions are classified as monovalent (one charge) or bivalent (two electrical charges). When the water is removed, through evaporation for example, the sodium and chloride ions will attract each other again and form salt crystals again. Molecules are groups of atoms which are held together by chemical bonds. Molecules themselves can become ions too. In our case sulfate (SO42-), for example, is an ion which consists of one sulfur and 4 oxygen atoms. It also carries 2 negtive charges. concentration, hardness and alkalinityBefore we can get into the units found in water reports we need to understand the different types of measurements that are found it water reports. First off, there are simple concentrations of substances, mostly ions, which are generally listed as mg/l or ppm. Hardness is a parameter which expresses the calcium and magnesium content of the water. Its name has little to do with actual changes in the water hardness but stems from the fact that the presence of calcium and magnesium makes it “harder” later soap lather. This is the result of a precipitating reaction between the soap, the calcium and the magnesium ions in the water. The resulting precipitate is soap scum and the higher the calcium and magnesium content of the water the more soap is consumed by the formation of soap scum. Water hardness is defined as the concentration of calcium and magnesium (technically any bivalent ion but only calcium and magnesium tend to be present it a significant amount). This combined concentration is not measured as mg/l or ppm since calcium and magnesium ions have different weights. Instead it is reported either as mEq/l or an equivalent amount of calcium carbonate (ppm as CaCO3). Water low in calcium and magnesium is called “soft” water while high calcium and magnesium concentrations make for “hard” water. Hardness may also measure only the calcium or magnesium concentration. In these cases it will be called calcium or magnesium hardness respectively. Alkalinity is a measure of the water’s pH buffering capacity and defined as the amount of acid equivalents it takes to lower the water’s pH to a defined end point. Most commonly this end point is a pH of 4.3 [deLange]. Since the major pH buffer in water is the carbonate system the water’s alkalinity can be used to determine the bicarbonate and carbonate concentration and vice versa. Like hardness, alkalinity is always given using an equivalent unit (mEq/l or ppm as CaCO3). About the units found in a water reportThe analysis results found in a water report may be given in a variety of units. What they mean and how to convert between them goes a long way in understanding a water report. mg/l or ppmFor all intents and purposes the units mg/l (milligram per liter) and ppm (parts per million) are and can be used interchangeably since one liter of water weighs 1 million milligrams. This unit is commonly used to measure the concentration of individual ions. Occasionally ug/l (microgram per liter) or ppb (parts per billion) are also used but rarely for the ions that we brewers are interested in.
mmol/lThough rarely found in water reports mmol/l (millimol per liter) is a very useful unit for chemical calculations. Rather than expressing the weight of a substance the chemical unit mole expresses the number of atoms, ions or molecules. To be exact 6.0221415×1023 atoms, ions or molecules make one mole. This is the same amount as there are atoms in 12g of carbon. [2] A millimol (mmol) is a thousandth of that. To convert between mg and mmol the weight of each atom, ion or molecule of the substance needs to be known. Those numbers have been published in chemical reference books but can also be calculated if the atomic masses of the individual atoms of a molecule are known. The internet provides a great reference for that. Example 1: The water contains 100 mg/l calcium. One calcium atom has an atomic weight of 40.08 g/mol. Since that weight is largely concentrated in the atom’s nucleus a calcium ion, which misses 2 electrons, is assumed to have the same weight. Hence 100 mg/l equals 100/40.08 = 2.5 mmol/l Eample 2: The water contains 100 mg/ bicarbonate (HCO3-). The molecular weight of bicarbonate is not known but the atomic weights of the individual atoms are known: H: 1 g/mol, C: 12 g/mol and O: 16 g/mol. The molecular weight of bicarbonate is the sum of its atoms: 1 + 12 + 3x16 = 61 g/mol. Hence the water contains 100/61 = 1.64 mmol/l bicarbonate. mEq/lThough mmol/l does express the number of ions, which works better for chemical calculations, it does not take the electric charges of these ions into account. The latter is useful for calculating neutralizing reactions between acids and bases for example as well as calculating the ion balance. The ion balance is the sum of all positive and all negative changes. They need to be equal in any realistic water composition, but more about this later. An equivalent (Eq) is the moles of ions multiplied with the number of electrical charges each ion carries. 1 mEq is one thousandths of that.
In some water reports mEq/l is the unit used for hardness and/or alkalinity although, in the US, ppm as CaCO3 is a more common unit. Equivalent unitsIn water reports it is common to express the amount of a particular mineral or group of minerals as the equivalent amount of another substance. Those units are very similar to mEq/l and will be discussed here as ppm CaCO3Measurements commonly expressed in ppm as CaCO3 are hardness and alkalinity. If a water sample has a hardness of 100 ppm as CaCO3 it has the same hardness the calcium that the calcium from 100 mg/l calcium carbonate would add. If it has an alkalinity of 100 ppm as CaCO3 it has the same acid neutralizing power 100 mg/l CaCO3 would have. One mole of calcium carbonate weighs 100g and its ions, Ca2+ and CO32-, have 2 electrical charges each 1 mEq/l equals 50 ppm as CaCO3. grainsdegrees German HardnessDegree German Hardness is an equivalent unit which is, as its name suggests, widely used in Germany. One degree of German Hardness (1 dH) is the hardness created by 10 mg calcium oxide (CaO) in one liter of water. The molaric weight of CaO is 56 g/mol and therefore 10 mg/l CaO equal 0.17 mmol/l or 0.35 mEq/l since the calcium ion has 2 positive charges. Thus: 1 dH = 0.35 mEq/l = 17.9 ppm as CaCO3 degrees French HardnessThe French defined their hardness on the basis of calcium carbonate. One degree French Hardness (1 TH) is the hardness added by 10 mg calcium Carbonate (CaCO3) in 1liter water. This unit is very similar to ppm as CaCO3 except that it is 1/10th of that: 1 TH = 10 ppm as CaCO3 = 0.2 mE/l = 0.56 dH Unless you are reading French water reports it is unlikely that you’ll ever encounter this unit. converting between the various unitsThe following table shows how to convert mg/l for the various ions found in water to mmol/l or the equivalent units discussed earlier. <add a table for converting from mg/l to mmol/l and mEq/l> reading a water reportNow that you have a water report and an understanding of the units used in it you take a closer look. These are the ions and water parameters that are of interest: Total HardnessThe total hardness (also called general hardness or GH) is an indication of the calcium and magnesium content. In most water’s about 70% of the hardness comes from calcium with the remaining 30% coming from magnesium. This can be used to estimate the calcium and magnesium content if those two ions are not listed separately Total Hardness calcium magnesium ppm as CaCO3 mg/l mg/l 25 50 75 100 150 200 300 400 Calcium (Ca2+)Atomic weight: 40.078 g/mol Calcium is an important ion for the brewing process and the main contributor to water hardness. It stabilizes alpha-amylase during mashing (although this enzyme still fairly stable at the temperatures that are commonly used for mashing) and aids in the protein coagulation [3]. It’s reaction with malt phosphates lowers the mash pH, an effect that is captured in the concept of residual alkalinity. Calcium promotes hot break flocculation <ref=name”Noonan”> Gregory J. Noonan, New Brewing Lager Beer, Brewers Publications, Boulder CO, 1996</ref> and is necessary for yeast flocculation where it becomes part of the bridges that are formed between yeast cells. A calcium content of more than 50 mg/l is recommended for preventing the excessive formation of calcium oxalate (beer stone) which can be the cause of gushing. Although the recommended concentration for calcium is between 50 and 150 ppm [4] I have been able to brew excellent clear lagers with as little as 30 ppm calcium. In general water’s used for lager brewing tend to contain less hardness (i.e. calcium and magnesium) than waters used for ale brewing. Magnesium (Mg2+Atomic weight: 24.305 g/mol Though Magnesium is an important co-factor for various enzymes during fermentation and also supports peptidase enzyme activity during mashing. Its presence in the water is not required since the malt adds on the order of 130 mg/l (4 l/kg mash thickness) [3]. Magnesium content in water of up to 50 mg/l is seen as acceptable. High magnesium levels combined with high sulfate levels can lead to the formation of magnesium sulfate (Epsom Salt) which imparts the beer an unpleasant bitterness. Similar to calcium magnesium also reacts with malt phosphates to lower the mash pH. But its effect is only about half that of calcium. Sodium (Na+)Atomic weight: 22.9897 At levels above 150 mg/l sodium can, together with chloride, lend the beer a salty taste. Below these concentrations sodium chloride can increase the beer’s mouthfeel [3]. Brewers aim to keep the sodium levels below 50 ppm Cite error: Invalid High sodium levels in water can be the result of water softeners which replace calcium and magnesium ions with sodium ions. Such waters are generally unsuitable for brewing. Five Star’s 52 mash buffer, for example, is largely a sodium phosphate salt which also adds sodium to the mash and later the beer. Potassium (K+)Atomic weight: 39.0983 g/mol While the malt adds potassium on the order of 500 mg/l to the mash the potassium content of the brewing water should be less than 10 mg/l [3]. other metal ionsIron causes problems at concentrations as low as 0.2 mg/l by inhibiting sacharification, increasing color of the wort and promote beer staling processes [3]. Excessive iron can also give the beer a metallic blood-like taste. Iron is part of the secondary (i.e. cosmetic) water standards and its level is recommended to be below 0.3 mg/l [5] Manganese is important for enzymes in yeast metabolism but excessive amounts cause the same problems as excessive iron content. The EPA recommends a manganese concentration of 0.05 mg/l or lower Cite error: Invalid Many other metals like copper, zinc, lead and tin are toxic to yeast when present in higher concentrations but important for yeast metabolism and growth at lower concentrations [3]. However, for successful fermentation none of these metals have to come from the brewing water. In the case of beer the malt is providing an adequate amount of these trace elements. bicarbonate/carbonate (HCO3-/ CO32-Molecular weight: 61.01 g/mol (HCO3-); 60.01 g/mol (CO32-) Bicarbonate and carbonate, which are the [#pH overview|conjugate bases] of carbonic acid, are responsible for the water’s pH buffering capacity which is expressed in its alkalinity. As a result of that they can be used to predict the water’s alkalinity. Other ions that have pH buffering properties are phosphates which are generally not present in significant amounts. The increased pH buffering brought on by bicarbonate and carbonate works against the acidity of the grist and can lead high mash and boil pH values. The effect of high mash and boil pH has been discussed in [#pH in brewing] If the water also contains significant amounts of calcium, carbonate is generally not present in any significant amount since it forms a poorly soluble salt wit calcium: calcium carbonate or chalk which eventually precipitates from the water. As a result the water pH tends to be below 9 at which point bicarbonate is the dominant ion that represents the water’s alkalinity. SulfateThe sulfate ion (SO42-) affects the perception of bitterness. High sulfate levels give the beer a dry and more bitter taste [Narziss&Back]. ChlorideThe chloride ion is known to enhance the activity of the alpha-amylase [Narziss&Back], [6] by binding to the enzyme and transforming it into a more active state [7]. Used as calcium chloride it gives the beer a full and smooth taste. Sodium chloride enhances the beer’s mouthfeel [Narziss&Back]. Chloride dominated waters tend to be better for malt dominated beers while waters high in sulfate are more suitable for hoppy and more bitter beers.
|
- ↑ Ground and drinking water, epa.gov
- ↑ Wikipedia, Mole (unit)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Ludwig Narziss, Werner Back, Die Bierbrauerei Band 2: Technologie der Würzebereitung, Wiley-VCH, 2009
- ↑ John J. Palmer, How to Brew, Brewers Publications, Boulder CO, 2006
- ↑ EPA, Secondary Drinking Water Regulations
- ↑ C. Eyester, The Optimum pH For Diastase Of Malt Activity, 1959, The Ohio Journal Of Science
- ↑ A. Hengge, Faculty, Chemistry and Biochemistry, Utah State University, [http://www.madsci.org/posts/archives/2000-04/956616009.Bc.r.html post on www.madsci.org