Understanding Efficiency
-----------------------------------work in progress-------------------------------------------------
Efficiency is a commonly discussed subject among all grain brewers. But with the abundance of definitions for it, it easily becomes a matter of comparing apples with oranges. This article tries to shed some light on the various efficiency definitions that are in place, how they are defined (sometimes differently, depending on the author) and how efficiency is affected.
Contents
Existing Definitons
In "How To Brew", John Palmer defined the brewing efficiency as the ratio between the gravity points of the wort in the kettle and the maximum potential (labratory extract) of the grain. The maximum potential of the grain is given in gravity units per pound and gallon. Based on that the gravity points of the kettle wort are [Palmer 2005]:
kettle gravity points = brewing efficiency * grain amount in pound * kettle volume * potential of the grains
When grains with different potential are used, the weighted average of their potential needs to be used in the above equation.
In "Designing Great Beers", Ray Daniels defines what John Palmer calls brewing efficiency mash efficiency [Daniels, 2000].
In "Abriss der Bierbrauerei", German brewing author Ludwig Narziss defines Sudhausausbeute (German for brew house efficiency) as the ratio between the amount of extract that made it into the boil kettle vs. the amount of grain that was used [Narziss, 2005]:
Sudhausausbeute = (kettle volume in l * kettle extract in % * kettle specific gravity) / grain mass in kg
Note that this is a different approach for defining the efficiency. The reference is not the maxium amount of extract that can be extracted from the grain, but the total weight of the grain. The latter includes the weight of the husks and other insoluble material. Because of that the the Sudhausausbeute is also affected by the potential (or laboratory extract) of the used grains. This is also the definition that German home brewers use for efficiency. Thus care needs to be taken when reading efficiency numbers from German sources. While 75% is a very good efficiency number when based on the total grain weight (most grains laboratory extract is about 80% of their weight) it is only a modest efficiency when it was based on the laboratory extract of the grain.
When asked how to calculate efficiency, the BYO Wizard replied with the same definition as was given in Narziss [BYO]. He calls that efficiency the brewhouse efficiency. But he also goes on and defines the efficiency that is based on the laboratory extract of the grain as brewhouse yield:
brewhouse yield = (kettle volume in l * kettle extract in % * kettle specific gravity) / (grain mass in kg * fine grind extract in %)
Furthermore, technical brewing articles oftentimes make mention of the Overall Brewhouse Yield (OBY). This is is defined like the BYO Wizard defined the brewhouse yield. It is affected by milling, mashing and lautering and basically indicates how close these brewhouse processes came to the fine grind laboratory extract.
The Handbook of brewing lists the total grain weight based efficiency (see Sudhausausbeute above) as the brewhouse yield. It furthermore mentions that the volume used in the equation is the volume of the hot wort in the kettle corrected for temperature. It only considers losses in the spent grain (unconverted starches and sugars held in the wort trapped in the grain) and not transfer losses on the way to the fermenter (e.g. wort loss in the trub) [Priest, 2006]. From this I gather that commercial brewers see the efficiency into the kettle as the brewhouse efficiency or yield.
Another popular set of efficiencies are the efficiency numbers given by Beersmith, a recipe design software. There 3 different types of efficiency are given: brewhouse efficiency based, efficiency into boiler, efficiency into fermenter. The brewhouse efficiency indicates how much of the extractable extract made it into a wort with the measured gravity and the the target volume that has been entered for that batch. Efficiency into boiler is the percentage of extractable extract that made it into the boil kettle. This is based on the entered pre-boil volume and pre-boil gravity. Lastly the Efficiency into fermenter is the percentage of extractable extract that ended up in the fermenter. For that the measured gravity in the fermenter and the wort volume in the fermenter is measured. The efficiency that matters for comparison with others is the efficiency into boiler or the brewhouse efficiency if the target volume matches the temperature corrected post boil volume. Any other efficiency measurement is not readily comparable because of losses that happened after the lautering process. One of the major losses is wort left behind in the kettle and its cause is fairly obvious.
extraction and lauter efficiency
The brewhouse efficiency can be broken into two separate efficiencies that measure the performance of mashing and lautering separately:
brewhouse efficiency = exttaction efficiency * lauter efficiency
Extraction efficiency measures how well the mash extracted the extract from the grain. If all the potentially extract (laboratory fine grind extract) has been extracted, the mash efficiency is 100%. Extraction efficiency is affected my mash parameters like pH, crush, diastatic power, temperature profile and mash time and should be close to 100%.
Lauter efficiency measures how well the lautering procedure did in transferring the extract, made soluble by mashing, into the boil kettle. It is affected by the design of the lauter system, type of lautering (no sparge, batch sparge and fly sparge) and amount of sparge water used. The parameters that affect the lauter efficiency for batch sparging have been discussed in Batch Sparging Analysis.
Extraction efficiency
Measuing extraction efficiency
Splitting brewhouse efficiency into extraction and lauter efficiency only helps in evaluating the brewhouse efficiency if one of the components can be measured separately. To determine the extraction efficiency it is best to calculate the theoretical maximum of the first wort extract/gravity based on the laboratory extract of the grain that was used and the volume of water that was added to the mash.
expected FW extract = grain mass * grain laboratory extract / (mash water volume + grain mass * grain laboratory extract)
- expected FW extract is the theoretical maximum of the extract content of the first wort in Plato (actually weight %, but that is close enough to Plato and Brix for these calculations)
- mash water volume is the volume of the strike water in liter. This means the total volume of water that was added to the mash before the FW extract is determined, including water that was added after the mash but before the first run-off. But water added to compensate for decoction boil-off should not be considered.
- grain mass is the grain weight in kg
- grain laboratory extract the (weighted) average of the laboratory extract of the grains. This comes from the malt analysis, but 0.8 is a fairly accurate estimation for most malts.
The mash efficiency is then the ratio between the expected FW extract and the actual FW extract (not exactly true, but close enough for these calculations)
extraction efficiency = 100% * actual FW extract / expected FW extract
- extraction efficiency is the efficiency of the mash in %
- expected FW extract is the expected first wort extract that was calculated above (in Plato, Brix or %)
- actual FW extract is the actual first wort extract that was measured (in Plato, Brix or %)
The extract content in Plato (close enough to Birx and extract % for these cases) can be estimated form specific gravity by using this formula:
Plato = (SG - 1.000) * 1000 / 4
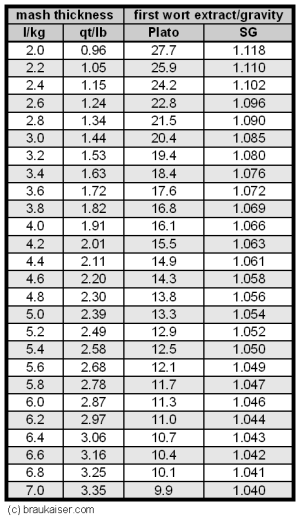
The first wort extract can also be calculated from the mash thickness, which removes the actual grain weight and water volume from the equation:
expected FW extract = 100 * grain laboratory extract / (R + grain laboratory extract)
- R is the water to grain ratio in l/kg. If the mash thickness is known in qt/lb, multiply by 2.11 to get it in l/kg
Table 1 gives the expected first wort extract/gravity readings based on the mash thickness at the time that the sample is pulled. To simplifiy the calculations this table assumes a 80% potential extract content in the grist (which is typical for most base malts) and 100% mash efficiency. Use these numbers as a benchmark to compare your measured first wort gravity against.
What affects the extraction efficiency ?
If the mash efficiency is significantly short of 100%, i.e. lower than 90%, the mash didn't perform as well as it should have. This is an indication that one or more of the mash parameters were suboptimal. To be precise mash parameters don't have to at their optimum for 100% extraction efficiency, they only have to be good enough. What is good enough for one mash parameter depends on the other mash parameters.
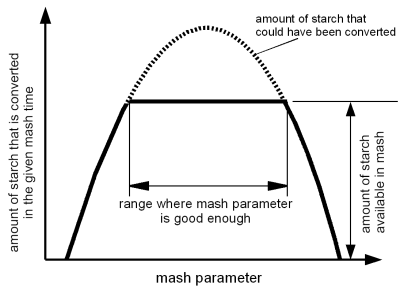
The reason for that good enough is the fact that the amount of starch to be converted is limited. And once that starch is converted and made soluble it doesn't matter how close a mash parameter was to its optimum there isn't more that can be converted. The extraction efficiency will plateau. This is illustrated in Figure 1
Lets assume the mash parameter in question is temperature and the mash time is 60 min. If the temperature is to low, the enzymatic activity will not be strong enough to convert all the starch in the mash within 60 min, as a result the extraction efficiency will suffer. But if the temperature is higher, the enzymes will be active enough to convert all the starch in the mash. At this point 100% extraction efficiency can be achieved. Even if the temperature is optimal and allows enzymatic activity that could convert twice the amount of starch, the extraction efficiency will not go up as it is limited by the amount of starch in the mash. At higher temperatures there comes a point where the denaturation of the enzymes is so strong that not all the starch can be converted anymore. From this point on the extraction efficiency will suffer as the rest temperature is increased.
In addition to that, other mash parameters my be far enough from their optimum that even an optimal temperature will not be able to convert all the starch in 60 min. As a result of that 100% extraction efficiency cannot be achieved at any rest temperature.
Besides resulting in less than 100% extraction efficiency, a shortfall in mash conversion can also be detected though a Starch Test. This test uses the reaction between iodine and long chains of glucose (which starch is) to detect the presence of starch in the mash liquid and the spent grains.
Temperature
As shown in the Limit of attenuation experiments, the rest temperature has an affect on fermentability and extraction efficiency. Generally it can be said that lower temperatures require longer rests in order to get the mash fully converted. This is a result of increased enzymatic activity of the enzymes. How long it takes to convert a mash at a given temperature depends on the other mash parameters. Temperatures higher than 75-80 C (167-176F) may cause to much of the alpha amylase (the main starch converting enzyme) to be denatured to quickly and thus resulting in a mash that may never convert.
A conversion below the starch gelatinization temperature (60-65 C / 140-150 F for the large starch granules which represent 85-90% of the starch and 51-92 C / 125 - 200F for the small granules that represent the rest of the starch [Briggs, 2004]) conversion still takes place but at a slower pace since the enzymes only have access to the starch on the outside of the starch granules. Once the starch gelatinizes the enzymes have access to much more starch hence conversion occurs much quicker. Given a constant rest time, there will be a rest temperature at which the mash is not able to fully convert in the given time and as a result efficiency will suffer. This can be compensated by a longer rest or getting other mash parameter closer to their optimum to strengthen the diastatic power of the mash.
pH
The amylase enzymes have a pH optimum between 5.4 and 5.7 when the pH of a cooled mash sample is measured (5.4-5.6 pH for a-amylase and 5.6-5.8 pH for beta amylase [Narziss, 2005]). This was also confirmed in the limit of attenuation experiments. Outside this pH range the enzymes still work, but not as well and the mash doesn't convert as quickly or, if the rest time is not long enough, won't convert and the extraction efficiency will suffer. Because of this, and for beer quality, a brewer should pay attention to the mash pH and/or the residual alkalinity of the brewing water. The residual alkalility, which is a function of the water's calcium, magnesium and bicarbonate content, affects how low the acidity of the malt will be able to lower the pH. Besides a chemical reaction between the malt and the water's calcium and magnesium ions (see Understanding Mash pH)melanoidens present in the malt also have an acidic power which lowers the pH. As a result grists of darker malts require water with higher residual alkalinity than grists of lighter malts to create a mash pH that falls into the optimal mash pH range of 5.4-5.7 (when measured at room temperature).
Many brewers see a jump in brewhouse efficiency once they correct the mash pH. This is the result of improved extraction efficiency. On information about how to estimate and correct mash pH see Understanding Mash pH.
Time
The longer the enzymes can work, the more they can convert. Hence a longer mash time can lead to an increase in extraction efficiency. But if the mash already fully converts before the rest time is over, an increase in the rest time will not have a significant affect on the extraction efficiency since there is nothing left to be made soluble by the enzymes.
This assumes that the temperature is low enough and doesn't cause excessive denaturation of the enzymes (at least for the alpha amylase). If the rest temperature cased to many enzymes to be denatured before full conversion was reached, no increase in the length of that rest will be able to fix the conversion problem. Only the addition of fresh malt or enzyme preparations can convert the mash now.
Malt Milling
How tight the malt has been crushed can have a big impact on the extraction efficiency. If the grits are to coarse and pieces of endosperm are still (partially) enclosed by the husks, the mash needs to be more intense to reach the starch inside these grits. As a result the extraction efficiency is likely to suffer. As the crush gets tighter, the size of the endosperm pieces is reduced and more of them are separated from the husks. The amount of flour also increases. There will be a point at which the largest pieces are small enough that intensity of the chosen mashing schedule is strong enough to reach and convert all the starch. Non stirred single infusion mashes are least intense. The "intensity" is increased if a step mash is used, the mash is stirred or even boiled as it is the case for decoction mashes.
Note that the malt grain is about 1.8 - 4.5 mm (0.07 - 0.18 in) thick. If it is crushed with a mill gap spacing of 1.0 - 1.5 mm (40 - 60 mil), which is the factory setting of many mills, it cannot be expected that there will be a sufficient separation between the endosperm and the husks and small enough grits that a single infusion mash is strong enough to reach all the starch. As a result many home brewers see a jump in efficiency when they start milling the grain through a tighter roller spacing or double crushing the grain.
But there is also a lower limit to the roller spacing. As the malt is crushed ever tighter the husks are shredded more and more (although that can be mitigated though Malt Conditioning) and more flour is produced. Both impede the lauter process and a stuck mash becomes more likely. If even a mill gap spacing as low as 0.6 mm (24 mil) doesn't achieve an extraction efficiency close to 100%, attention should be paid to the other mash parameters. Most likely one or more other mash parameters are suboptimal and reduce the "strength" of the mash.
In general it is best to crush as tight as necessary for close to 100% extract efficiency (full conversion) but not tighter as to improve the run-off speed of the lauter and avoid excessive husk shredding.
Mash Schedule
As it was already alluded to in Malt Milling the intensity of the mash also affects how well a mash converts. That "intensity" is determined how the mash is performed. Triple decoction mashes are regarded as the most intense mashes since the boiling of the grain liberates starch that is still enclosed in cell walls (undermodified malts) or tucked away between husk pieces (poor malt crush) and makes it accessible to the enzymes. But on the other hand, decoction mashes also reduce the diastatic strength of the mash by denaturing enzymes during the decoction boils. Another increase in intensity is stirring and agitating of the mash. But modern well modified malts generally don't need an intensive mashing schedule and if the other mash parameters are otimal (or good enough) a mash with these malts can convert even without decoction mashing or constant stirring.
Dough-in at temperatures below the saccrificantion rest temperatures helps to preserve the diastatic power because the amylase enzymes are able to hydrate before they enter a temperature range in which they start to denature more significantly.
When starches like rice or corn, which have higher gelatinization temperatures, are used it might be necessary to gelatinize these starches before they are added to the mash. Flaked grains had that already done and can be added directly raw rice or corn need to be cooked to accomplish that. In breweries that use adjuncts the vessel used for this boil is called a cereal cooker.
Diastatic Power
Diastatic power is a measure of the enzymatic strength (in particular the amylase enzymes) of the malt. The higher that power is, the more amylase enzymes are in the mash and the more starch can be converted by these enzymes and the more forgiving the mash can be if other mash parameters are suboptimal. Diastatic power is measured in degree Linter or W-K (Windisch-Kolbach units). To determine it, gelatinized starch is incubated with a water extract made from the malt and kept at a controlled temperature. After a predetermined time, the starch breakdown is measured and it seen as an indication of the enzymatic strength of the malt [Briggs, 2004]
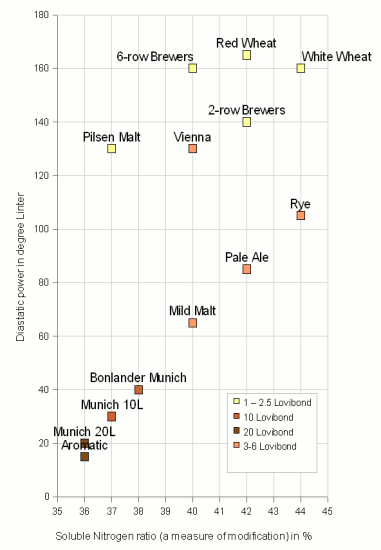
The diastatic power of malt is affected by the germination and kilning process during the malt production. While a longer germination time (higher degree of modification of the malt) increases the number of enzymes and diastatic power a higher kilning temperature (darker malts) reduces the diastatic power by denaturing a larger number of them. Figure 2 shows the dependency between malt color and the diastatic power of the malt for malts from Briess Malting. The darker the malt, the higher and/or longer the kilning process and thus the lower the diastatic power. It is interesting to note that the relationship is not linear but falls rather quickly as the malt color increases. As a result only ~20% of Pilsner malt (130 deg. Linter) need to be added to a grist of 100% dark Munich (20 deg. Linter) to double its diastatic strength while the color of the grist (now 20% Pilsner and 80% Munich) only decreases by 20%. This should be kept in mind when designing recipes. If a grist is suspected to be to weak in diastatic power, it doesn't take much lightly colored malt to correct that. In the case of Briess malts, it would be even better to use Vienna malt instead of the Pilsner malt as it is darker colored but has the same diastatic power as the Pilsner malt.
The reason that the Vienna malt has a higher diastatic power is that it was produced with a longer germination time which lead to a higher modification and high soluble nitrogen ratio. The longer malt is left to germinate, the more enzymes are produced (increase in diastatic power) and the more protein (nitrogen compounds) are degraded and made soluble (increase in soluble nitrogen vs. total nitrogen i.e. soluble nitrogen ratio SNR or S/T). This relationship can be seen in Figure 2. Among malts of smimilar color, it is in general true that the higher the soluble nitrogen ratio, the higher the diatatic power. Some exceptions do however exist. The Briess Vienna malt for example has the same color as the Pale Ale malt and a lower SNR as the Pale Ale mat, yet its diastatic power is significantly higher.
While all this sounds complicated, most modern malts have a sufficient diastatic power to be used in a single infusion mash. According to ??? the lower limit is ??? Linter (find that number). But with respect to efficiency and full mash conversion, the closer the grist's diastatic power is to that limit the less forgiving will the mash be with respect to other mash parameters having to be at their optimum.
Another reduction of diastatic power happens during decoction mashing. While decoction mashing makes the starch more accessible to the enzymes by gelatinizing the starch present in the decoctions, it also destroys the enzymes that were present in the decoction. As a result special care should be taken when decocting mashes weak in enzymatic power (i.e. large amounts of dark base malts like Munich malt). To mitigate the problem of the reduction in enzymatic power decoctions should be rested for conversion before they are brought to a boil. This uses the power of the enzymes that are to be destroyed soon for the starch that is present in the decoction and as a result not as much enzymatic power is needed during the saccrification rest of the main mash. If possible decoction mashes should be kept thin (3.5 to 5 l/kg / 1.75 to 2.5 qt/lb) and the decoctions itself thick (about 2 l/kg or 1 qt/lb is a good thickness for a thick decoction). This keeps more of the enzymes, which are after dough-in quickly dissolved in the mash liquid, in the main mash while the decoction will get more of the starch and other grain material. In addition to that monitor the conversion process with the Starch Test. If conversion is slow, a rest at 70 - 74 C (160 - 168F) can speed things up. If that is not working add some (~10%) Pilsner or Pale malt can be added to add more enzymes.
Mash thickness
In order to convert the starches, water is needed. Not only for process of gelatinization or hydration of the enzymes but also for the conversion process itself. Whenever a gluclose chain is split (either to create a sugar molecule or a shorter starch chain) one molecule of water is needed. In addition to the reduced amount of free water the high sugar concentrations in thick mashes also the amylase enzymes [Briggs, 2004].
In the limit of attenuation experiments it was shown that a 5 l/kg (2.4 qt/lb) mash showed much better extraction efficiency than a 2.5 l/kg (1.2 qt/lb) mash.
Dough Balls
Dough balls form when the gelatinized starch encloses dry starch/malt. The gelatinized starch forms a barrier that keeps mash water from entering the dough ball. The same happens in cooking when you are trying to thicken hot liquid by adding corn starch or flour. You will likely create clumps that take some whisking to be broken up. Because of that the starch or flour should be mixed with some cold water first before the mix is added to the hot liquid.
In brewing dough balls can be avoided by slowly adding malt to hot strike water and stirring well after the malt is added. Thinner mashes are also less likely to cause dough balls. No risk of dough balls exists when the dough-in happens below the starch gelatinization temperature of 60 C (140F). In contrast to the flour clumps in cooking, the enzymes of the mash will start working on the barrier of gelatinized starch and eventually break that barrier. That leads to a late release of starch which may or may not be converted in time (dependent on the enzymatic strength of the mash) which affects the extraction efficiency negatively and as a result dough balls should always be avoided through careful mash-in and thorough stirring of the mash after dough-in. Make sure you are also getting into the corner of rectangular mash tuns.
Lauter efficiency
The lautering process (i.e. separation of sweet wort and spent grain) is the other process that affects the brewhouse efficiency. Other than mashing it is a mostly physical process. In home brewing 2 strategies are employed: batch sparging (including its extreme the no-sparge) and fly sparging. While batch sparging relies on the contrinued dillution of the wort left in the grain after a run-off by batches of sparge water, fly sparging is a continuous process in which the sparge water leaches and rinses the extract from the grain.
Estimating Lauter Efficiency (Batch Sparging)
In the batch sparging process, after the mash is complete, the first wort is run off until the grain bed runs "dry". But it is not really dry and a volume of wort (of the same gravity as the wort that was run off) remains between the grains. Now the valve is closed and a batch of sparge water is added to the grain and stirred in. That dilutes the wort that remained in the grains and once the stirring is done another batch of wort, which is lower in gravity this time, is run off into the boil kettle. This process is repeated until the desired pre-boil volume is reached. One to two batches of sparge water are used most often.
Because of the static nature of this problem run-off -> dilution -> run-off ... the lauter efficiency of batch sparging can easily be modeled. The efficiency of transferring dissolved extract (sugar, proteins etc.) is the ratio between run-off volume and total wort volume in the mash tun. Because of the presence of undissolved solids, the wort volume in the lauter tun is not equal to the mash volume and it is better to calculate the wort volume in the lauter tun as the sum of run-off volume and volume absorbed by the grain and the wort dead spaces that cannot be drained. In a properly designed lauter tun, the wort absorbed by the grain is generally much larger than the wort trapped in dead spaces. That volume is also dependent on the amount of grain that was mashed.
The efficiency of one batch sparge step is:
- Effbatch_sparge_step = 100% * mextract_kettle / mextract_lauter_tun
- Effbatch_sparge_step = 100% * e * Vrun_off / (e *(Vrun_off + Vgrain_absorption + Vdead_space))
e is the extract content (i.e. gravity) of the wort. It is the same for the divisor and dividend and can be eliminated from the equation.
- Effbatch_sparge_step = 100% * Vrun_off / (Vrun_off + Vgrain_absorption + Vdead_space)
This is also the efficiency of a no-sparge. In the subsequent discussions the dead space volume will be neglected, but if it is significant it needs to be added to the volume absorbed by the grain.
To calculate the percentage of extract (efficiency) that a subsequent batch sparge lauters from the grain additional amounts of "extract" need to be added to the dividend of that equation. The dividend corresponds to what has been run off into the kettle. But when the mash is run off again, the initial gravity of the wort has been diluted and the wort that is now run into the boil kettle is worth less in terms of efficiency. This is accounted for with the dilution factor Vgrain_absorption/(Vrun_off + Vgrain_absorption) which is the ratio between the wort held back in the grain and the total wort volume in the lauter tun after adding the sparge water and before running it off.
- Eff2_step_batch_sparge = 100% * (Vrun_off + (Vgrain_absorption/(Vrun_off + Vgrain_absorption))*Vrun_off) / (Vrun_off + Vgrain_absorption)
As the number of run-offs ever weaker worts are run into the kettle and ever smaller amounts are added to the dividend. The result is a diminishing increase in efficiency.
Measuring Lauter Efficiency (Batch and Fly Sparging)
While mathematical modeling works well for batch sparging, it doesn't work so well for fly sparging due to the dynamic nature of the process. This doesn't mean that it has been done in commercial brewing, especially by equipment manufacturers, in search for ever more efficient lauter tun designs, but there are to many parameters that affect lauter efficienct wich are difficult to measure or keep constant that developing a mathematical model for fly sparging is not a practical option for the home brewer.
Instead a method of estimating the fly sparging efficiency by measuring the dissolved extract, that remains in the spent grain after the run-off is complete, exists. This method is not restricted to fly sparging, it also works for batch sparging. The idea is to dilute the wort that is in the spent grain and measure its gravity/extract. The volume of wort after the water addition can be estimated by the wort absorption rate for the grain and the amount of water that was added. Based on that volume and gravity/extract, the weight of the dissolved extract left in the spent grain can be determined and the ratio between that weight and the initial extract weight in the grain are the brew house efficiency percentage points that were lost during the lauter.
- mextract_left_in_lauter = (Vwater_added - Vgrain_absorbed) * sg * extract
The amount absorbed by the grain can be calculated from the total amount of water used, the amount of wort produced and the amount of extract that was extracted from the grains. The latter is important as it actually increases the volume by about 0.62 l for each kg of dissolved extract. A common absorption ratio for grains is 1.56 l/kg or 0.19 gal/lb.
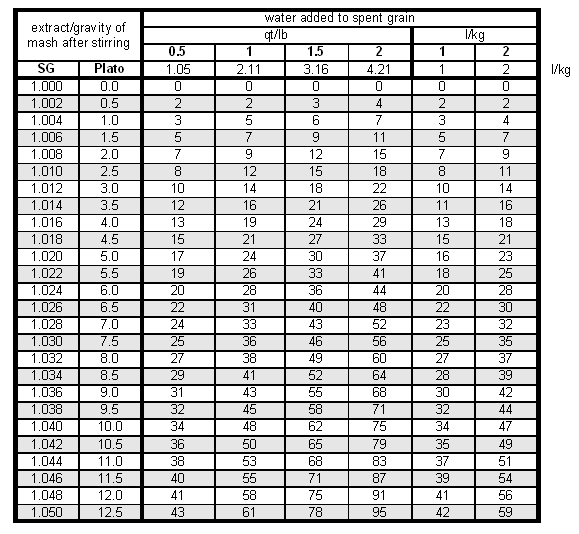
If an extract potential of 80% is assumed for the grain and the amount of water added is expressed as multiples of the grain weight, the following formula can be used to determine the brewhouse efficiency loss during the lauter based on the added water and gravity of the mash liquid after thorough stirring.
- Efflost_in_lauter = extract * sg * (Agrain_absorption + Rwater_added) / 0.8
The grain absorption rate (Agrain_absorption) of 1.56 l/kg and the water addition rate (Rwater_added) need to be entered as l/kg. Table 2 has been created using the above formula and Testing the Lauter Efficiency in Troubleshooting Brewhouse Efficiency shows how to calculate the lauter efficiency from the efficiency loss in the lauter tun.
What affects Lauter Efficiency in batch sparging
The parameters, that affect the batch sparging efficiency, have been discussed in detail in Batch Sparging Analysis. The following is just a brief review
Grist Size / Starting Gravity
These two are related to each other. The higher the starting gravity of the beer should be, the the more grain is needed in the mash. In batch sparging, the grist size affects the efficiency by increasing the divisor in the basic batch sparging efficiency equation:
- Eff = 100% * Vrun_off / (Vrun_off + Vgrain_absorption)
The more grain is used the more wort will be held back by the grain (Vgrain_absorption) and the lower the efficiency will be. This is the reason why big beers, which generally use more grain per given volume of water, have a lower batch sparge lauter efficiency than smaller beers.
Number of run-offs
While it is obvious that each additional sparge step (run-off) will bring more of the extract from the mash into the boil kettle and thus increase the efficiency,
Preboil volume / boil-off
Having a larger preboil volume (which will require a larger boil-off to get to the desired post boil extract/gravity) increases the lauter efficiency by increasing the dividend in aforementioned equation. In simple terms, the more water that is available for rinsing out the extract, the higher the lauter efficiency will be. But the boil-off should only be changed within a limited range. Excessively long boil times or excessively strong boils (hourly boil-off of 15% or more) can be detrimental to the beer quality.
What affects Lauter Efficiency in fly sparging
Sources
- [Palmer, 2006] John J. Palmer, How to Brew, Brewers Publications, Boulder CO, 2006
- [Daniels, 2000] Ray Daniels, Designing Great Beers, Brewers Publications, Boulder CO, 2000
- [BYO], Online article Gravity & Brewhouse Efficiency
- [Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan), Abriss der Bierbrauerei. WILEY-VCH Verlags GmbH Weinheim Germany, 2005
- [Briggs, 2004] Dennis E. Briggs, Chris A. Boulton, Peter A. Brookes, Roger Stevens, Brewing Science and Practice, Published by Woodhead Publishing, 2004
- [Briess 2008] Briess average malt analysis data 2007/2008
- [Priest, 2006] F. G. Priest, Graham G. Stewart, Handbook of Brewing, CRC Press, 2006