The Science of Mashing
------------- Work in progress ------------
In mashing the milled grain (grist) is mixed with water to create the mash. It is an essential process in the production of beer and a continuation of the malting process on the way to sweet wort. During the mash soluble malt compounds like enzymes, proteins and sugars are dissolved by the mash water and insoluble malt compounds like starch and some long chained proteins are converted into soluble compounds and dissolved into the water. The latter happens through a combination of physical and biochemical process which can be controlled by the brewer to achieve a sweet wort of desired quality. The biochemical processes are catalyzed by various malt enzymes. Their function and behavior is dependent on the conditions in the mash (e.g. temperature, pH, concentration, etc.) and the brewer should be familiar with that behavior in order to control the quality of the sweet wort that is run off from the mash in the lautering process.
Contents
Enzymes
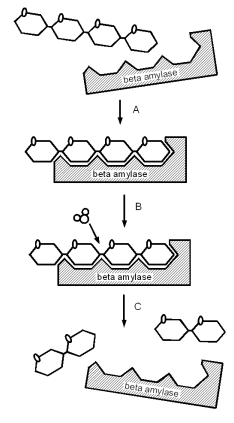
Enzymes are very important to mashing. They catalyze conversion reactions which break down malt compounds (the largest one being starch). In the case of starch this conversion is necessary to form water soluble dextrines and sugars. The latter of which can be metabolized by the yeast. Enzymes are proteins (chains of amino acids) which have the ability to lower the energy needed for a chemical chemical reaction which allows that reaction to occur faster and at lower temperatures. Most enzymes are very specific to the reaction they catalyze and work only with a specific substrate and produce only a specific product. In case of the beta amylase enzyme, for example, the substrate is a glucose chain and the product is maltose. The reaction that is catalyzed the the split of a glucose chain link while a molecule of water is consumed. The highly specific nature to the reaction that is catalyzes stems form the shape of the enzyme which is just right for reacting with the substrate and releasing the product and residual product shortly after. The enzyme itself is not consumed in the reaction. I.e. once free again it can catalyze another reaction.
Without an enzyme a chemical reaction may look like this [rsc.org]:
- substrate1 + substrate2 -> product
But the initial energy (i.e. temperature) needed for this reaction may be so high that it rarely ever happens. With the presence of an enzyme this reaction changes to
- substrate1 + enzyme -> enzyme-substrate-intermediate
- enzyme-substrate-intermediate + substrate2 -> product + enzyme
The energy needed for these enzymatic reactions are now low enough for the reaction to happen at practical temperatures (e.g. mash temperatures).
Here is an eample of an actual enzymatic reaction in the mash. The enzyme is maltase which can split a maltose molecule into two glucose molecules. The reaction consumes one water molecule:
- C12H22O11 + H2O -> 2 C6H12O6
With the enzyme maltase the reaction is actually 2 reactions:
- C12H22O11 + <Maltase> -> C12H22O11-<Maltase>
- C12H22O11-<Maltase> + H2O -> C6H12O6 + <Maltase>
The same is true for other enzymatic reactions in the mash. But for simplicity sake the shorter form of:
- substrate1 + substrate2 -> product
Is also used to show the reaction that is happening. The involvement of the enzyme in this reaction is implied
As mentioned earlier. Enzymes are generally very specific in the type of substrate they work on and which reaction they catalyze. A simple model for that behavior is the lock-and-key hypothesis [rsc.org]. In that theory, the enzyme has a shape that fits only a particular substrate. Like the beta amylase which fits only on the non reducing end of a starch chain (Figure 1). If the shape of the enzyme changes significantly due to temperature or pH or inhibitors block the receptor site of the enzyme it cannot react with the substrate and the enzyme becomes inactive.
How temperature effects enzymatic reactions
The rate of most chemical reactions, including enzymatic reactions, follows the Arrhenius equation [wikipedia.org]:
- reaction rate = A * e(-ΔG*/RT)
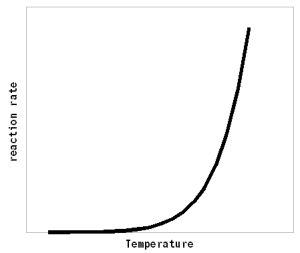
Where A is the Arrhenius constant, a simple non exponential factor for the reaction rate. ΔG* is the activation energy of the reaction and determines at what temperature the reaction will become "significant". R is the universal gas constant and T is the absolute temperature in Kelvin. To convert a temperature in Celsius into Kelvin 273.15 needs to be added to the Celsius value (I.e. the absolute temperature (0 Kelvin) is -273.15 Celsius). But all that doesn't really matter for understanding this subject. What matters is that the reaction rate of an enzymatic reaction follows an exponential curve (Figure 2). For every 10 C (18F) increase the reaction rate increases 1.2 - 2.5 fold [lsbu.ac.uk].
But if the enzymatic reaction rate follows an exponential curve, why are there different temperature optima for different enzymes?
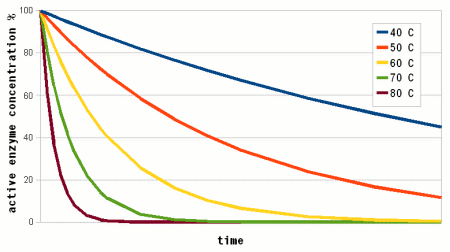
To answer this question we need to look at another effect that temperature has on enzymes. As mentioned earlier, enzymes are proteins (long chains of amino acids) that are shaped in a particular way which allows them to hold onto their substrate during the reaction. This shape is held in place by weak bonds between different amino acids of the chain. But these bonds are easily broken if the temperature gets too high and the enzyme molecule starts to vibrate too much. This doesn't cause the chain of amino acids to get split, these bonds are much stronger and require much higher temperatures, but the enzyme looses its shape and becomes unable to catalyse the reaction it was made for. It is said to have denatured. Above a critical temperature the rate of denature increases 6 - 36 fold for every temperature increase of 10 C [lsbu.ac.uk]. This is much higher than the increase in the reaction rate and as a result, the productivity of the enzyme will decrease is the temperature is raised past the temperature optimum. Figure 3 shows how the enzyme concentration of a hypothetical enzyme drops for 5 different temperatures.
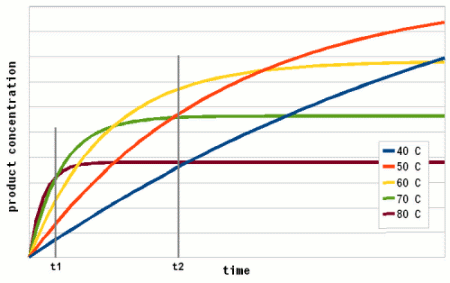
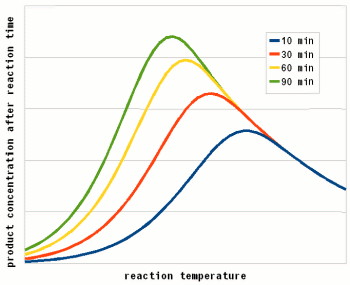
Based one the rate of reaction (Arrhenius equation) and the drop of the active enzyme concentration over time (Figure 3) one can plot the increase of product if unlimited substrate is available. Having unlimited substrate available is not a realistic scenario for mashing but it helps in showing the relationship between the temperature optimum of an enzyme and the reaction time. Figure 4 shows the graphs for the rise in product concentration over time for a hypothetical enzyme at 5 different temperatures. While a high temperature causes a steep initial rise in temperature this high temperature also causes a quick drop in the enzyme concentration and the rise in product concentration quickly levels off and falls behind lower reaction temperatures.
A simple analogy to this behavior is a toy car powered by an electric motor rated for 10V. If the motor is run at 5 volt the car will run very slow but may be able to run for a long time. If run at 10V the car will go faster but only until the motor reached the lifespan it was designed for. Run at 15 V the car will be even faster but the motor is likely to burn out shortly. And if run at 20V the car will get the fastest start of all but won't get far since the motor will burn out quickly. The same happens with the enzymes. At high temperatures they are sprinters and at lower temperatures they are marathon runners.
So far the discussion has only been for virtually unlimited supply of substrate in comparison to the number of enzymes that are available. But this won't be the case in mashing where for example only a limited amount of starch will be available for the amylase enzymes. In this case the temperature optimum changes to a temperature that is good enough to reach full conversion. After that there is no more substrate available and that particular enzymatic reaction will come to a halt. Before that point is reached, the enzymatic reaction will also start to slow down because it becomes harder for the enzymes to find substrate to react with.
Inhibitors and cofactors
Some enzymes require the presence of a non protein compound, commonly a metal ion, for the reaction. Without this cofactor the enzyme is not able to catalyze the reaction it was made for. As a result the rate of the enzymatic reaction may also be limited by the concentration and availability of the necessary cofactors. One important cofactor for enzymes needed by the yeast metabolism is for example zink.
Inhibitors reduce the reaction rate of the enzymes.
The effect of pH
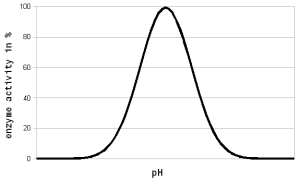
The pH of a solution is a logarithmic measure of the freely available H+ ions. The concentration of these ions affects the enzymes in various ways. A change in pH loads to changes in the charges on the enzyme due to the reaction of the H+ ions with the carboxyl or amino functional groups [cuny.edu].
The at high pH (low concentration of H+ in the solution> the carboxyl group disassociates
- COOH → COO- + H+
This leaves a negative charge at the site of the carboxyl group and donates a proton H+ to the solution. This reaction is reversed at low pH
At low pH (high concentration of H+ in the solution) the amino functional group binds a proton
- NH2 + H+ → NH3+
This results in a positive charge on the site of the amino group. A reaction that is reversed at high pH.
These pH dependent reactions determine the electric charges and as a result the shape of the enzyme and its ability to react with the substrate. As a result a pH exists at which the enzyme will be most efficient performing the reaction it was designed for. On either side of that optimum the effectiveness of the enzyme will decline until it is unable to function anymore.
The change in enzyme activity within 2-3 pH units to either side of the optimum is generally a reversible process [lsbu.ac.uk]. This means that no permanent damage is done to the enzyme. It also means that a suboptimal pH in mashing doesn't necessarily damage the enzymes and that the full enzymatic activity can be restored by adjusting the mash pH into the optimal range, except for the enzymes that have already been denatures. A more severe pH change 3+ units is likely to denature the enzyme due to high stress that the extreme change in the charges place on the structure of the enzyme. This basically makes the enzyme less stable and it denatures at a fairly high rate even below its critical temperature. In some cases strong acidity can also hydrolyze the peptide links (i.e. break the amino acid chain the makes up the enzyme).
When considering pH, it should be noted that the pH of a solution changes with temperature. The rate of change is dependent on the solution itself. For wort and mashes the actual pH is 0.35 units lower at 65 C (150F) than at room temperature 20 C (68 F). At 75 C it is 0.45 units lower [Briggs, 2004].
- [rsc.org] Chemistry for Biologists - How enzymes work
- [lsbu.ac.uk] The effect of temperature on * [lsbu.ac.uk The effect of pH on enzymes, London Southbank University
- [wikipedia.org] Arrhenius Equation
- [cuny.edu] pH and enzymes, City University of New York
Starch
- where it is found
- glucose and 1-4/1-6 branches
- amylose
- amylopectin
- starch granule structure
Starch conversion
- gelatinization
- active enzymes
- emzyme temperature and pH optima
- mash parameters affecting conversion