Building brewing water with dissolved chalk
|
In order to be able to build brewing water with a profile that matches a particular city or to build any brewing water that has temporary hardness chalk (CaCO3) is needed. Chalk, however, is not very soluble in water and most of what needs to be added remains suspended or settles to the bottom. ContentsAbout chalk and the carbonate systemIf you do not want to be bothered with the chemistry behind dissolving chalk you can safely skip ahead. Chalk CaCO3 is the a salt of carbonic acid. It's ions are calcium (Ca2+) and carbonate (CO32-). If we know one thing about chalk in brewing it is that it doesn't dissolve very well in water. This is because the solubility product between calcium and carbonate is a very small number. Chemists write it like this:
The names in brackets ([]) stand for the concentration of the particular ion. What that formula means is that a solution can only hold calcium and carbonate in solution as long as the product of their concentrations is less or equal to 3.36×10-9. If the product is greater calcium carbonate will precipitate until enough calcium and carbonates have been removed from the water to satisfy the equation. But how can we get calcium carbonate (a.k.a chalk) dissolved in water? We do this by lowering the carbonate concentration. To understand this we have to take a look at carbonic acid and the carbonate system. Carbonic acid is formed when carbon dioxide is dissolved in water. Not all the carbon dioxide will form carbonic acid but some does according to this chemical equation:
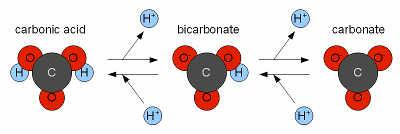
The "↔" means that there is a constant back and forth between both sides. I.e. carbonic acid is formed and falls apart into CO2 and water constantly. But on average there is always a portion of CO2 bound as carbonic acid. The interesting thing about carbonic acid is that it is a weak acid that can loose up to two protons (H+). We talked about weak acids in Weak Acids and Bases where we saw that the loss of the protons or acceptance of protons depends on the pH of the environment. When a carbonic acid molecule looses one proton it becomes a bicarbonate ion:
The process is reversible. I.e. if a bicarbonate ion picks up a proton it becomes carbonic acid. When a bicarbonate ion looses a proton it becomes carbonate:
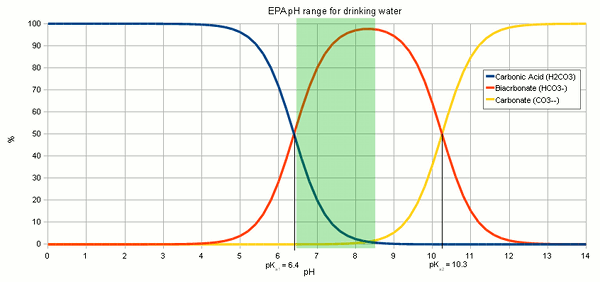
That process is illustrated in Figure 1. As mentioned earlier, the relative concentration of these 3 carbonate species, as they are called, [deLange] depends on the pH since it is pH that determines how much H+ are floating around. The more there are the more carbonic acid will exist and the less there are the more carbonate will be present. Figure 2 plots these relative concentration as % of the total carbonic acid + bicarbonate + carbonate amount over a pH range of 0 to 14. But how does this tie into dissolving chalk? Well, earlier we saw that we need to lower the concentration of carbonate in order to allow chalk to dissolve and the way to do that is to convert the carbonate that is already in the water (either present or from a small amount of chalk that already dissolved) into bicarbonate. That process consumes protons. It is these protons that we have to supply to dissolve more chalk than the water can hold without any help.
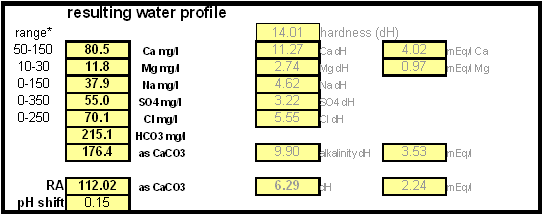
While chalk can be dissolved in water using acids, it defeats the purpose since the use of acids counteracts the alkalinity that the chalk is supposed to give the water. Only when chalk is dissolved by using CO2 is it able to raise the alkalinity of the water. The addition of CO2 creates carbonic acids which is able to convert the chalks carbonate ion (CO32-) to a bicarbonate ion (HCO3-). While doing that the carbonic acids itself is converted to bicarbonate. In the end one dissolved molecule of chalk (CaCO3) contributes 2 bicarbonate ions (HCO3-) and 1 calcium (Ca2+) ion to the water. To brew an upcoming batch of Kaiser Alt I want to mimic the water profile of the city of Düsseldorf where most of the Alts are brewed in Germany. One of the most well known Alt breweries there, Zum Uereige, does not use city water. According to this on-line statement the brewing water for that brewery comes from their own deep well. The Düsseldorf public water however is essentially cleaned and treated water from the river Rhein. The current water report for Düsseldorf is available on-line: Water report Düsseldorf and upon calculating the residual alkalinity for that water one finds 108 ppm as CaCO3 or 6.1 dH (dH is German Hardness). Such a residual alkalinity is well suited for brewing a dark amber beer like an Alt and I assume that most Alt breweries use city water without any water treatment. | ||||||
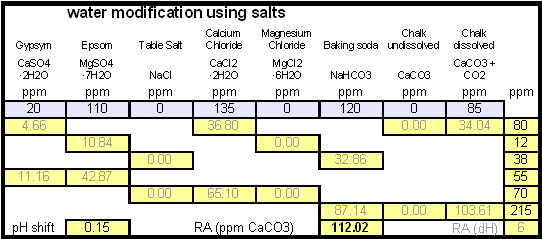
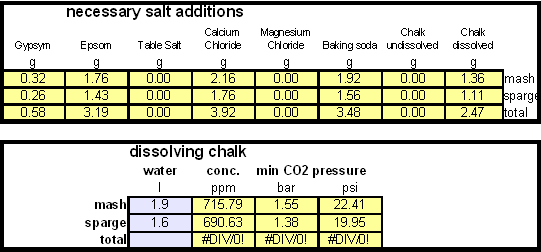
Calculating the salt additionsIn order to calculate the addition of dissolved chalk correctlt you need a brewing water spreadsheet that supports dissolved chalk. Most of the available spreadsheets don't since they assume that each chalk molecule contributes only one bicarbonate ion. My water spread sheer, Kaiser_water_calculator.xls supports both dissolved and undissolved chalk in brewing water. In general, when dissolving chalk only half the amount of chalk is needed to build waters with the same residual alkalinity. |
| |||||
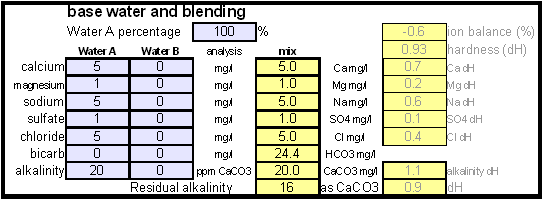
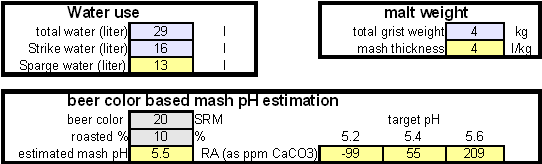
Step 1On the "advanced" work sheet enter the starting water profile. I use reverse osmosis water which is very soft water and for which I have a water analysis. If you are using reverse osmosis water and don't have an analysis simply leave the fields for the starting water profile 0. Due to the low ion concentration compared to the final ion concentration of the brewing water the error will be minimal and to small to matter. Step 2With an eye on the resulting water profile (section below or right most column) enter concentrations of brewing salts. Rather then entering the final amounts of salts needed and considering the volume of water that will be treated salts are entered as ppm (equivalent to mg/l). This makes the "water recipe" independent of the volume of water that needs to be treated. Once the water volume is known the spreadsheet calculates the required amounts of salts and displays them in a later section. Step 3Check the resulting water profile. If you compare this profile to the Düsseldorf water report that was referenced earlier you'll find it to be a very close match. This is not always possible since we brewers don't have all the combinations of cations (positive ions) and anions (negative ions), i.e. salts, available to us. In those cases focus on matching the residual alkalinity and calcium, sulfate and chloride content of the water. Step 4Now it is time to enter the total water volume and the strike water volume. The spreadsheet will calculate the sparge water volume from these numbers. You should also enter the grist weight which will allow for the the calculation of the mash thickness. The mash thickness is important for making a mash pH estimation from the beer color and the residual alkalinity since mash thickness determines how much pH shift a given residual alkalinity can cause. Once you enter the beer color in SRM and how much of the specialty malts are roasted malts an estimate of the mash pH can be made. In this case it is 5.5 and well within the acceptable range of 5.2 - 5.7. The lower right hand corner of the mash pH section also shows estimates for the residual alkaliniies that would be necessary to reach a mash pH of 5.2, 5.4 and 5.6 respectively. The large range from ~-100 to ~200 ppm as CaCO3 shows how wide of a range of brewing waters could be used to brew a 20 SRM beer with a mash thickness of 4 l/kg (~2 qt/lb) Step 5Using the water volumes specified earlier, the amounts of salts needed can now be calculated. Because I don't have the ability to treat all the water at once I have to treat mash and sparge water separately. But for those able to treat all the needed water at once the amounts needed for the total volume are also given. Of interest for dissolving chalk is the "dissolving chalk" section. Here you enter the volume of water that the chalk should be dissolved in and the spreadsheet calculates the CO2 pressure that is needed to dissolve the chalk. You'll quickly find out that the relation between chalk concentration and pressure is not linear and that the pressure quickly shoots up once you exceed about 750 ppm. As a result this is generally the practial limit and to dissolve more chalk you need to dissolve it in a larger mount of water. If possible I like to dissolve my chalk additions in 2 l soda bottles and in this case it will be possible. Using 1.9 l to dissolve the mash water addition and 1.6 l to dissolve the sparge water addition I need only 1.55 bar and 1.38 bar respectively. Those pressure numbers are absolute which means that they include atmospheric pressure and that I have to use >0.55 bar and >0.38 bar as the setting on the regulator. A CO2 pressure regulator measures the pressure in excess of the atmospheric pressure. Another practical means of dissolving chalk are corny kegs. They hold a much larger water amount and as a result will require less CO2 pressure to dissolve chalk. Confirming that the chalk has been dissolved is more difficult, since it requires pulling a sample. Building the brewing water
| ||||||