Alkalinity reduction with slaked lime
|
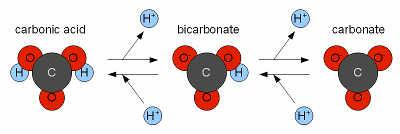
High alkalinity in their brewing water keeps many home brewers from being able to brew lighter beers. To overcome this obstacle home brewers would use reverse osmosis water to dilute their brewing water or even build their brewing water from scratch through the addition of salts. Another option is the use of acids to neutralize some or all of the water's alkalinity. If most or all of the water's alkalinity is the result of temporary hardness (dissolved calcium and magnesium carbonate) another options exists which is commonly overlooked: alkalinity precipitation using lime. Due to its low cost this method of brewing water treatment is widely used in the brewing industry but home brewers appear to have largely stayed away from it due a lack of understanding of the chemistry behind it and missing support in brewing water calculators. Based on A.J. deLange's work [deLange] and through pointers in technical brewing literature [Narziss&Back,2009] I was able to get it to work in my brewery and add support for the necessary calculations to the water calculation spreadsheet (Kaiser_water_calculator.xls). The following article attempts to shed light on this process and provide a better understanding of the chemistry behind it which hopefully allows other brewers to get this to work with their water in their brewery. ContentsA unintuitive conceptMost brewing books mention that the alkalinity of brewing water can be lowered by the addition of a lime (calcium hydroxide) which itself is a strong alkali. This always confused me and it took a while until the chemistry behind this process became clear. To better understand the chemica process described here the reader should have read An Overview of pH as well as About chalk and the carbonate system. Calcium hydroxide is a strong base and as such it completely dissociates into calcium and hydroxide when it dissolves in water:
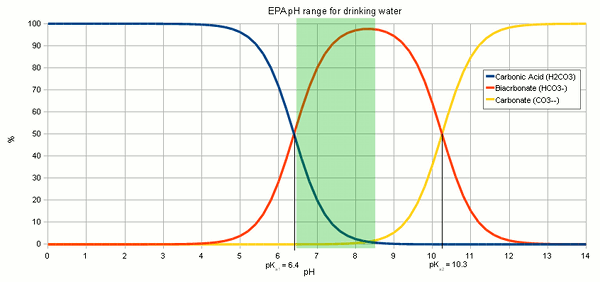
The resulting hydroxide ions (OH-) want to raise the pH of the solution and in pure water will be able to that very effectively. Hoever, the water we are treating with lime contain bicarbonate and carbonic acids with act as a pH buffer and stand in the way of a dramatic pH rise. While we have experienced alkalinity only as a hinderence for lowering the pH of the mash a pH buffer works both ways. I.e. it keeps the pH from falling as much as as it keeps it from rising. Since the ratio of carbonic acid, bicarbonate and carbonate stands in close relation to the pH of the solution (Figure 1) the hydroxide ions from the lime need to react with the carbonic acid (H2CO3), carbon dioxide (CO2 that hasn't formed carbonic acid yet) and bicarbonate (HCO-3) to increase the concentration of carbonate (CO2-3) through the following reactions
The added hydroxide basically sucking the Hydrogen ions (H+) from both the carbonic acid and bicarbonate to covert them to carbonate. This corresponds to traversing to the right in Figure 2. At some point most of the hydroxide ions have been consumed by aforementioned reactions and the carbonic acid/bicarbonate/carbonate balance is such that it is biased heavily towards carbonate. This went along with a rise in pH since the relationship between pH and carbonic acid/bicarbonate/carbonate, which is shown in Figure 1, has to remain satisfied. But what matters is not the risen pH but the increased concentration of carbonate ions. This is when we have to look at the solubility equation for calcium carbonate which has already been mentioned in About chalk and the carbonate system:
If the product between the concentration of calcium and carbonate ions is greater than 3.36×10-9, which is a very small number, calcium carbonate will precipitate out of solution until either the calcium concentration or the carbonate concentration is low enough to satisfy this equation. When this happens a milky white haze forms which will slowly settle.
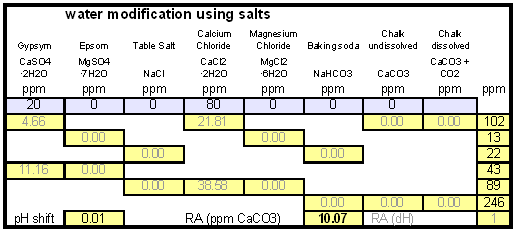
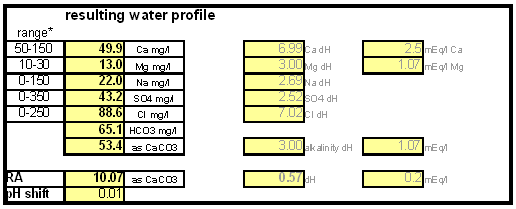
The down arrow (\u2193) means that the formed chalk is precipitating. This precipitating chalk represents the temporary hardness of the water and the clear water that remains has a lower alkalinity than it had before LimitationsThis kind of water treatment can only reduce alkalinity if sufficient calcium is present since it relies on the formation of calcium carbonate. Though changes can be made to the procedure (see #Magnesium reduction) the amount of magesium that can be removed with this method is limited due to the fact that magnesium carbonate is about 100 times more soluble than calcium carbonate [uri.edu]. Sodium cannot be reduced with this method since sodium carbonate is very soluble in water. What cannot be reduced either are sulfate and chloride anions. Those whill remain unchanged unless more are added though the addition of calcium salts, a pratice that is commonnly used to boost the water's calcium content and ensure an adequate calcium concentration in the treated water. Since alkalinity is only precipitated to the same extend that alkalinity is precipitated it is important that the calcium hardness exceeds the alkalinity. Calcium hardness is just another way of expressing the calcium content of the water. Rather then using the weight of the calcium, which is done when using ppm or mg/l as unit, the calcium content is represented as an equivalent amount of CaCO3 for example. To convert the calcium content from mg/l or ppm to ppm as CaCO3 simply multiply with 2.5. Eaxmple: The water contains 100 ppm calcium and has an alkalinity of 200 ppm as CaCO3. How much calcium remains after all alkalinity has been precipitated? The calcium hardness of that water is 250 ppm as CaCO3. Removing 200 ppm worth of alkality in the form of precipitated chalk also requires that the calcium hardness is reduced by 200 ppm. The remaining calcium hardness is 50 ppm as CaCO3 or 20 mg/l. Because of the calcum precipitation it might be necessary to supplement the water with calcium using calcium carbonate or gypsum. This is best done before or together with the addition of the lime. The amount needed depends on the expected calcium precipitation and the targeted calcium level of the post-treatment water. All of the calcium that is added by the lime will also be precipitated in the form of calcium carbonate. In pratice this method is not able to completely reduce the alkalinity to 0 regardless of the amount of calcium that is present on the water. The practical lower limit of alkalinity reduction is 30-50 ppm as CaCO3. One reason for that is absorption of atmospheric CO2 during the settling time which causes some of the precipitating chalk to be dissolved again. How to use the spreadsheetHow to treat brewing water with limedetailed calculation for determining the necessary lime additionsThis section is intended for brewers who want to incorporate the lime treatment option into their own spreadsheet. It details the calculations that are necessary and is thus only for brewers wo want to know about that detail of the subject.
References
|