Difference between revisions of "At home water testing"
| Line 1: | Line 1: | ||
| − | {| style="width: | + | {| style="width:860px" |
| | | | ||
| Line 29: | Line 29: | ||
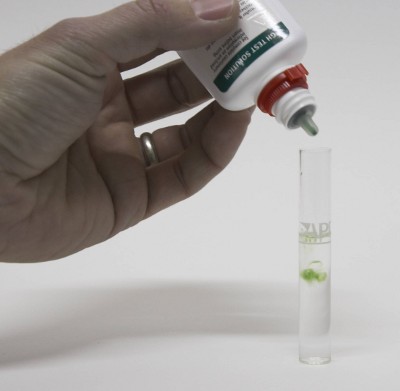
[[Image:KH_titration.jpg|frame|right|'''Figure 4''' - KH titration of the same water sample. It took 7 drops until the color changes from blue to green and later yellow. Thus the alkalinity of the water is 7 dH or 125 ppm as CaCO<sub>3</sub>]] | [[Image:KH_titration.jpg|frame|right|'''Figure 4''' - KH titration of the same water sample. It took 7 drops until the color changes from blue to green and later yellow. Thus the alkalinity of the water is 7 dH or 125 ppm as CaCO<sub>3</sub>]] | ||
| + | |||
| + | |||
| + | |- | ||
| + | | | ||
As mentioned earlier the process by which these tests work commonly known as titration. During a titration a chemical, titrant, is added to a sample of known amount. This titrant reacts with the compound to be tested until all of that compound in the sample has been consumed. An indicator signals that this point has been reached. Based on the amount of titrant that has been added the unknown amount of the compound in the tested solution can be calculated. | As mentioned earlier the process by which these tests work commonly known as titration. During a titration a chemical, titrant, is added to a sample of known amount. This titrant reacts with the compound to be tested until all of that compound in the sample has been consumed. An indicator signals that this point has been reached. Based on the amount of titrant that has been added the unknown amount of the compound in the tested solution can be calculated. | ||
| Line 37: | Line 41: | ||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
Revision as of 04:39, 6 December 2009
|
Water composition is important for brewing and many brewers either send their water to a lab for analysis for build their water from scratch by using very soft (e.g. reverse osmosis water) and salts. It is, however, also possible to test brewing water at home. The precision and amount of detail of such a water test at home does not match that of a professional analysis, but it is sufficient to estimate the residual alkalinity of the brewing water with an acceptable precision. At home water testing also allows regular testing of the water source in order to detect seasonal changes that may warrant a more precise professional analysis. Test kits Figure 1 - A typical GH&KH test kit for aquarium use. It contains test tubes, test chemicals and instructions. The total price for kits like these is around $6 and while there are more expensive test kits available for fish owners they are useless to brewers since they test water parameters that aren't of interest for brewing. To do that water testing we brewers can use water test kits that are available for aquarium owners. The kind of test kit that we need is one that tests GH and KH. GH stands for General Hardness and measures the total hardness of the water. In water speak the total hardness is the amount of calcium and magnesium ions present. It is commonly measured as either dH (German Hardness) or ppm as CaCO3. Both these units are equivalent measures. That measns that they don't express the weight of the calcium and the magnesium ions but their number multiplied by their electrical charge. The latter is 2+ for both of them. With knowledge of the atomic weight and an guess on the calcium to magnesium ratio commonly found in water one can estimate the calcium and magnesium content of the analyzed water [DeLange]. KH stands for Karbonat Härte (German for carbonate hardness) which is the alkalinity of the water. Like total hardness or GH it is measured as either German Hardness (dH) or ppm as CaCO3. The conversion of 1 dH = 17.8 CaCO3 is true for both total hardness and alkalinity. Two types test kits are available: titration based and strips. Titration based test kits contain test tubes and chemicals that are added until there is a color reaction in the water. Test strips are submersed in the water and the color reaction on their test pads is then compared against a color scale. While more difficult to use I prefer titration based tests since they provide more precise results are are easier to "read" than strips. Test procedureFor the exact instructions how to use the test kit read the instruction that come with it. In particular how to convert the amount of titrant, that has been added, to a hardness value in dH or ppm as CaCO3. But in general they all work the same:
| |
|
As mentioned earlier the process by which these tests work commonly known as titration. During a titration a chemical, titrant, is added to a sample of known amount. This titrant reacts with the compound to be tested until all of that compound in the sample has been consumed. An indicator signals that this point has been reached. Based on the amount of titrant that has been added the unknown amount of the compound in the tested solution can be calculated. In the case of an alkalinity test (KH test) a strong acid is added that consumes the carbonates and bicarbonates which establish the water's alkalinity. That reaction produces carbonic acids and more importantly lowers the pH. Once the pH as been lowered to about 4.3 virtually all carbonates and bicarbonates have been converted. At that point the indicator solution, which is nothing more than a pH indicator changes color from blue to yellow/green. The concentration of the test solution is designed such that each drop contains enough acid to neutralize 1 dH or 17.8 ppm as CaCO3 in 5 ml water. As a result the precision of the test can be increased by increasing the sample size to 10 ml and assuming that each drop stands for 0.5 dH or 8.9 ppm as CaCO3. A similar reaction takes place during testing for GH.
|
|
|
|