Difference between revisions of "Experiment Pitching Rate and Oxygenation"
(→Results and Discussion) |
(→Materials and Methods) |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
=Abstract= | =Abstract= | ||
| − | Esters are an important component of the aroma of German wheat beers. Common home brewing knowledge lists pitching rate and level of oxigenation as important factors that effect the level of esters that are produced during fermentation. While it is commonly believed among home | + | Esters are an important component of the aroma of German wheat beers. Common home brewing knowledge lists pitching rate and level of oxigenation as important factors that effect the level of esters that are produced during fermentation. While it is commonly believed among home brewers that lower pitching rates result in higher ester levels, the literature reports that increased pitching rates lead to higher levels of esters. This experiment is designed to evaluate the affect of oxygen levels and yeast pitching rate on the ester production. |
| + | |||
| + | While differences could be shown in the fermentation performance of the various fermentation samples, the taste and aroma of all the samples was remarkably similar which rendered this experiment inconclusive. | ||
=Introduction= | =Introduction= | ||
| − | Esters are formed through a condensation reaction between an alcohol and an acid [Wikipedia]. In brewing, 2 major processes exist in which esters are formed. During intra cellular ester formation, the yeast's metabolism produces esters through enzymatic reactions. But esters can also be formed by a simple condensation reaction between an organic acid and an alcohol. But due to the slow reaction rate, this form of esterformation doesn't play a role in primary fermentation and produces significant results only after extended aging (12+ weeks) [Hermann 2005 | + | Esters are formed through a condensation reaction between an alcohol and an acid [Wikipedia]. In brewing, 2 major processes exist in which esters are formed. During intra cellular ester formation, the yeast's metabolism produces esters through enzymatic reactions. But esters can also be formed by a simple condensation reaction between an organic acid and an alcohol. But due to the slow reaction rate, this form of esterformation doesn't play a role in primary fermentation and produces significant results only after extended aging (12+ weeks) [Hermann 2005, Narziss 2005]. This ester formation during aging is responsible for the dark fruit notes of aged beers. But more interesting for the brewing of Weissbier is the ester production during the primary fermentation. |
During Fermentation pyruvic acid, an intermediate product of the pathway that leads to alcohol, is reduced to oxalacetate and then to acetyl CoA. Acetyl CoA is the basis for a host of compounds including sterols for cell wall construction and esters [Noonan 1996]. All authors agree that increased biomass production (creation of cell walls) reduces the Acetyl CoA that is available for ester production and leads to reduced ester levels in the beer [Narziss 2005, Clone, Walsh, Noonan 1996, Fix 1999]. Differences however exist with respect to ester production and yeast growth. Fix [Fix 1999] writes that any increased activity on the acetyl CoA branch, i.e. yeast growth, will increase ester production while other authors [Narziss 2005, Clone] state that increased yeast growth leads to a decrease in esters since more of the acetyl CoA is used for sterol synthesis. | During Fermentation pyruvic acid, an intermediate product of the pathway that leads to alcohol, is reduced to oxalacetate and then to acetyl CoA. Acetyl CoA is the basis for a host of compounds including sterols for cell wall construction and esters [Noonan 1996]. All authors agree that increased biomass production (creation of cell walls) reduces the Acetyl CoA that is available for ester production and leads to reduced ester levels in the beer [Narziss 2005, Clone, Walsh, Noonan 1996, Fix 1999]. Differences however exist with respect to ester production and yeast growth. Fix [Fix 1999] writes that any increased activity on the acetyl CoA branch, i.e. yeast growth, will increase ester production while other authors [Narziss 2005, Clone] state that increased yeast growth leads to a decrease in esters since more of the acetyl CoA is used for sterol synthesis. | ||
| Line 11: | Line 13: | ||
The following is a list of the factors that are known to affect the ester production: | The following is a list of the factors that are known to affect the ester production: | ||
| − | * '''Yeast stain''': the choice of yeast strain has a significant impact on the easter production. But according to Narziss is a yeast strains ability to form esters strongly dependent on the wort composition and high ester levels in one type of wort are not necessarily an indocation that the same strain will also produce high levels of esters in another wort [ | + | * '''Yeast stain''': the choice of yeast strain has a significant impact on the easter production. But according to Narziss is a yeast strains ability to form esters strongly dependent on the wort composition and high ester levels in one type of wort are not necessarily an indocation that the same strain will also produce high levels of esters in another wort [Hermann 2005]. |
* '''availability of oxygen''': It has been shown that increased oxygenation rates can lower the amount of esters produced by yeast [Narziss 2005, Noonan 1996, Fix 1999]. This has been explained by the increased production of biomass. When free oxygen is available, the acyl CoA is more likely to be used for the production of sterols and the amount available for ester production will be reduced. | * '''availability of oxygen''': It has been shown that increased oxygenation rates can lower the amount of esters produced by yeast [Narziss 2005, Noonan 1996, Fix 1999]. This has been explained by the increased production of biomass. When free oxygen is available, the acyl CoA is more likely to be used for the production of sterols and the amount available for ester production will be reduced. | ||
| − | * '''Double batch (German: Drauflassen)''': As noted earlier, any process that keeps the yeast in the growth phase decreased ester production. One of these methods is | + | * '''Double batch (German: Drauflassen)''': As noted earlier, any process that keeps the yeast in the growth phase decreased ester production. One of these methods is Drauflassen or double batch brewing. A fresh batch of aerated wort is added to the fermenter when the previous batch is at high kraeusen. This can be done multiple times and the continued yeast growth will reduce the amount acyl CoA that can be used for ester formation [Narziss 2005]. |
* '''Temperature''': Higher fermentation temperatures are known to increase the production of fermentation byproducts including esters. Some authors have explained this with increased yeast metabolism. Saerens et. al. [Saerens 2007] gives this explanation "According to Suomaleinen [Suomalainen 1981], an increase in the fermentation temperature releases higher levels of esters through more efficient excretion and/or enhanced autolysis of the yeast. An effect of the temperature on the thermodynamic equilibrium of ester solubility in cellular lipids and the aqueous medium is another possibly more likely explanation". But it should be noted that the effect of temperature on the ester production is different between yeast strains and also vary between the types of esters that are produced [Hermann 2005] | * '''Temperature''': Higher fermentation temperatures are known to increase the production of fermentation byproducts including esters. Some authors have explained this with increased yeast metabolism. Saerens et. al. [Saerens 2007] gives this explanation "According to Suomaleinen [Suomalainen 1981], an increase in the fermentation temperature releases higher levels of esters through more efficient excretion and/or enhanced autolysis of the yeast. An effect of the temperature on the thermodynamic equilibrium of ester solubility in cellular lipids and the aqueous medium is another possibly more likely explanation". But it should be noted that the effect of temperature on the ester production is different between yeast strains and also vary between the types of esters that are produced [Hermann 2005] | ||
| − | * '''pressure''': Even though increased CO<sub>2</sub> pressure leads to the retardation of yeast growth, it has been shown to reduce the amount of esters that are produced. The increased CO<sub>2</sub> pressure is affecting the synthesis of acetyl CoA which results in retarded yeast growth and lower ester production [Hermann 2005]. This shows that if yeast growth is slowed by | + | * '''pressure''': Even though increased CO<sub>2</sub> pressure leads to the retardation of yeast growth, it has been shown to reduce the amount of esters (and other fermentation byproducts) that are produced. The increased CO<sub>2</sub> pressure is affecting the synthesis of acetyl CoA which results in retarded yeast growth and lower ester production [Hermann 2005]. This shows that if yeast growth is slowed by reduced acetyl CoA levels, the ester production will not be increased. |
| − | * '''pitching rate''': Higher yeast pitching rates lead to increased ester levels in the beer [Hermann 2005]. This statement conflicts with common home brewer knowledge [Fix 1999], that underpitching leads to less clean fermentations. According to Narziss, increased ester production is a result of the reduced yeast growth that results from high pitching rates. | + | * '''pitching rate''': Higher yeast pitching rates lead to increased ester levels in the beer [Hermann 2005]. This statement conflicts with common home brewer knowledge [Fix 1999], that underpitching leads to less clean fermentations. According to Narziss, increased ester production is a result of the reduced yeast growth that results from high pitching rates [Narziss 2005]. |
* '''FAN levels''': It has been shown that an increase in free amino nitrogen (FAN) leads to an increase in ester production [Saerens 2007]. Free amino nitrogen refers to the short length proteins that the yeast absorbs during fermentation. This leads to the conclusion that beers brewed with a high percentage of adjuncts (corn, rice) should show less ester production and that beers with a high FAN level (extended protoelytic activity during the mash. i.e. long protein rest at 50C) should contain more esters. | * '''FAN levels''': It has been shown that an increase in free amino nitrogen (FAN) leads to an increase in ester production [Saerens 2007]. Free amino nitrogen refers to the short length proteins that the yeast absorbs during fermentation. This leads to the conclusion that beers brewed with a high percentage of adjuncts (corn, rice) should show less ester production and that beers with a high FAN level (extended protoelytic activity during the mash. i.e. long protein rest at 50C) should contain more esters. | ||
| − | * '''wort composition''': The higher the percentage of maltose in the wort, the less esters will be produced. Hermann showed that an increase in the worts glucose concentration leads to an increased ester production. The necessary increase in glucose can be achieved through corn-sugar additions or mashing procedures that take advantage of the | + | * '''wort composition''': The higher the percentage of maltose in the wort, the less esters will be produced. Hermann showed that an increase in the worts glucose concentration leads to an increased ester production. The necessary increase in glucose can be achieved through corn-sugar additions or mashing procedures that take advantage of the maltase enzyme. This enzyme can create glucose from maltose, but is not active in typical mashing as it is deactivated above 45 C [Hermann 2005]. Hermann explained that with a delayed occurence of the diauxic point (when the yeast has to switch from glucose to maltose as their primary sugar source). This switch causes the yeast stress under which it produces more esters. If it is delayes, more yeast will encouter it due to the increased yeast growth that has happened up to this point [Hermann 2005]. |
* '''wort gravity''': The higher the wort gravity, the more esters will be produced during fermentation [Saerens 2007]. The higher wort gravity provides more compounds for enzymatic reactions, the length of the fermentation is increased and the increased sugar concentration inhibits yeast growt which leads to more Acyl-CoA for ester production [Hermann 2005] | * '''wort gravity''': The higher the wort gravity, the more esters will be produced during fermentation [Saerens 2007]. The higher wort gravity provides more compounds for enzymatic reactions, the length of the fermentation is increased and the increased sugar concentration inhibits yeast growt which leads to more Acyl-CoA for ester production [Hermann 2005] | ||
| Line 37: | Line 39: | ||
* wort composition (through mashing or sugar additions) | * wort composition (through mashing or sugar additions) | ||
| − | The objective of this experiment was to evaluate the qualitative effects of oxygenation and pitching rate on the aroma compounds created by a Weissbier yeast. | + | The objective of this experiment was to evaluate the qualitative effects of oxygenation and pitching rate on the aroma compounds created by a Weissbier yeast. |
=Materials and Methods= | =Materials and Methods= | ||
| − | For this experiment a 12 Plato wort was brewed from a | + | For this experiment a 12 Plato wort was brewed from a the following grist: |
| Line 48: | Line 50: | ||
| − | + | A Hochkurz mash with the following rests was used to mash the grain: | |
| Line 57: | Line 59: | ||
| − | The 25L preboil volume wort was then boiled for 60 min with 10g Target hops (10% a-acids). The wort was cooled to a pitching temperature of 17C. 9 | + | The 25L preboil volume wort was then boiled for 60 min with 10g Target hops (10% a-acids). The wort was cooled to a pitching temperature of 17C. 9 2 liter sanitized PET Pepsi Cola bottled were filled with 1.5 l each. The wort was then oxygenated using a sintered stainless steel oxygen stone and a regulator. Previously it was determined that the flow rate of oxygen from the oxygen stone at the chosen regulator setting is about 0.6 mg/s. After that the yeast was pitched. The used yeast originated from a pack of Wyeast 3056. I later realized that this is actually a blend and since I have been storing and propagating this yeast for a while I have no idea how the relationship between the yeast strains that were blended changed. The pitched yeast culture came from the slurry of a primary fermentation that started 2 weeks earlier. |
The fermentation samples were oxygenated and pitched as follows: | The fermentation samples were oxygenated and pitched as follows: | ||
| Line 68: | Line 70: | ||
| B | | B | ||
5.0 ml/l yeast slurry | 5.0 ml/l yeast slurry | ||
| − | + | 1 s oxygen (0.4 ppm) | |
| C | | C | ||
15 ml/l yeast slurry | 15 ml/l yeast slurry | ||
| − | + | 1 s oxygen (0.4 ppm) | |
|-align="center" | |-align="center" | ||
| D | | D | ||
| Line 94: | Line 96: | ||
|} | |} | ||
| − | The oxygenation times were taken from my standard | + | The oxygenation times were taken from my standard ale oxygenation time of 60s for 20l of wort. I later calculated the amount of O2 that was released from the stone, but not all of the released O2 was actually absorbed by the wort. In addition to that the head space also contained oxygen, even more than was added through the oxygen stone. As a result of that the oxygenation part of this experiment cannot be deemed reliable. |
The pitching rates amount to the following pitching rates for 20l wort: | The pitching rates amount to the following pitching rates for 20l wort: | ||
| Line 106: | Line 108: | ||
The fast ferment test showed a limit of attenuation of 78% (apparent extract = 2.6 Plato). | The fast ferment test showed a limit of attenuation of 78% (apparent extract = 2.6 Plato). | ||
| − | All samples were fermented in | + | All samples were fermented in a single water bath at an average temperature of 19 C (peaked at 20C on day 4). On day 6 the samples were bottled in 12 oz bottles with the addition of corn sugar for carbonation. The amount of corn sugar was based on the current extract level of the beer in order to achieve similar carbonation levels despite the fact that the samples had different extract levels at the time of bottling. |
The bottles were then stored at about 19C for 8 more days before they were moved to an ambient temperature of 15 C where they sat before sampling. | The bottles were then stored at about 19C for 8 more days before they were moved to an ambient temperature of 15 C where they sat before sampling. | ||
=Results and Discussion= | =Results and Discussion= | ||
| + | |||
| + | == Fermentation performance == | ||
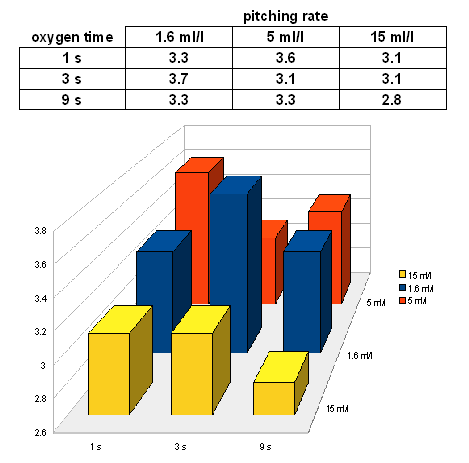
On day 5 the following extract levels were measured for the 9 samples: | On day 5 the following extract levels were measured for the 9 samples: | ||
| Line 116: | Line 120: | ||
[[Image:Weissbier_Experiment_I_5_day_extract.gif]] | [[Image:Weissbier_Experiment_I_5_day_extract.gif]] | ||
| − | With some outliers, the results | + | With some outliers, the results matches the expectation that higher pitching rates and more oxygen lead to a faster fermentation. The floor in the graph has been set to the extract level that was determined though the fast ferment test (2.6 Plato). |
| + | |||
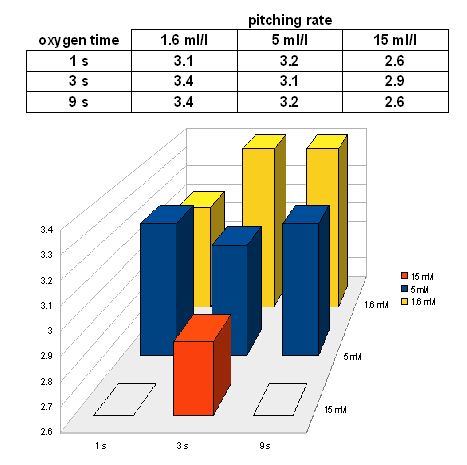
| + | On day 6 (bottling day), the extract levels were not as consistent | ||
| + | |||
| + | [[Image:Weissbier_Experiment_I_6_day_extract.gif]] | ||
| + | |||
| + | While the samples don't seem to follow the expected trend for oxygenation times, they seem to follow the expected trend for the pitching rates. Two of the samples with the highest pitching rate were able to reach the limit of attenuation (78% or AE = 2.6 Plato). The absence of a clear relationship between the oxygenation rate and the fermentation performance is assumed to be a result of the imprecise oxygenation which was discussed earlier. | ||
| + | |||
| + | = Taste test = | ||
| + | |||
| + | The following are notes on the aroma and taste tests that were done on the various fermentation samples. All taste tests were done by only one taster and none of the beers exhibited a strong ester profile to begin with. This was an indication that the used yeast may not have been the best for this experiment. | ||
| + | |||
| + | 12 days after bottling the samples A, B and C (lowest oxygneation, 3 different pitching rates) were evaluated for taste and aroma. It was notes that sample A was less estery than sample C but the difference was very sublte. Actually so subtle that three triangle blind tests between samples A and C, that were done in succession, did not yield a conclusive result. A slight "band aid" taste and aroma was also noted for sample A, but not strong enough for sample A to stand out in the triangle test. | ||
| + | |||
| + | 18 days after bottling, the samples G, H and I (highest oxygenation, 3 different pitching rates) were evaluated for taste and aroma. The results were similar to the first taste test and no conclusive difference was found in 3 triangle tests between G and I, though it was noted that H showed slight "band aid" aroma and taste immediately after pouring. | ||
| + | |||
| + | 20 days after bottling D, E and F (medium oxygen, 3 different pitching rates) were evaluated. Sample D (lowest pitching rate) showed a band aid aroma and taste, but besides this the aroma and taste between all three samples is very close and it was difficult to tell them apart. | ||
| + | |||
| + | 21 days after bottling B and H (medium pitching rate, lowest and highest oxygen) were compared. No "band aid" was found in either sample but B was regarded as less clean and appeared to have more fusel alcohols. But the taste and aroma of both samples was almost the same. No difference in the ester levels was perceived. | ||
| + | |||
| + | 31 days after bottling A (lowest oxygen and lowest pitching rate) was compared against I (highest pitching rate and oxygen). Both were remarkably close to each other but I seemed more "rounded" in its taste than A without being able to say exactly why. No significant "band aid" was found in either sample. | ||
| + | |||
| + | Given the fact that none of the performed blind tastings was able to definitively tell a difference between 2 beers, it certainly possible that the little differences that were perceived were influenced by the knowledge of which sample was tasted. | ||
=Conclusion= | =Conclusion= | ||
| + | |||
| + | Despite the 9 fold difference in pitching rate and oxygen injection, which showed differences in the fermentation performance, the samples tasted remarkably similar and none of the blind taste tests could tell them apart. The actual difference in dissolved oxygen that was available to the yeast was likely much smaller, but the pitching rates were definitely widely different. | ||
| + | |||
| + | This experiment needs to be seen as inconclusive and further experiments need to be done with a different yeast. But at that point only 3 or 4 fermentations should be run to test either the influences of the pitching rate or the oxygen levels. Having only 3 to 4 samples that need to be evaluated is more manageable than 9 samples. | ||
=Sources= | =Sources= | ||
Latest revision as of 18:14, 12 August 2008
Contents
Abstract
Esters are an important component of the aroma of German wheat beers. Common home brewing knowledge lists pitching rate and level of oxigenation as important factors that effect the level of esters that are produced during fermentation. While it is commonly believed among home brewers that lower pitching rates result in higher ester levels, the literature reports that increased pitching rates lead to higher levels of esters. This experiment is designed to evaluate the affect of oxygen levels and yeast pitching rate on the ester production.
While differences could be shown in the fermentation performance of the various fermentation samples, the taste and aroma of all the samples was remarkably similar which rendered this experiment inconclusive.
Introduction
Esters are formed through a condensation reaction between an alcohol and an acid [Wikipedia]. In brewing, 2 major processes exist in which esters are formed. During intra cellular ester formation, the yeast's metabolism produces esters through enzymatic reactions. But esters can also be formed by a simple condensation reaction between an organic acid and an alcohol. But due to the slow reaction rate, this form of esterformation doesn't play a role in primary fermentation and produces significant results only after extended aging (12+ weeks) [Hermann 2005, Narziss 2005]. This ester formation during aging is responsible for the dark fruit notes of aged beers. But more interesting for the brewing of Weissbier is the ester production during the primary fermentation.
During Fermentation pyruvic acid, an intermediate product of the pathway that leads to alcohol, is reduced to oxalacetate and then to acetyl CoA. Acetyl CoA is the basis for a host of compounds including sterols for cell wall construction and esters [Noonan 1996]. All authors agree that increased biomass production (creation of cell walls) reduces the Acetyl CoA that is available for ester production and leads to reduced ester levels in the beer [Narziss 2005, Clone, Walsh, Noonan 1996, Fix 1999]. Differences however exist with respect to ester production and yeast growth. Fix [Fix 1999] writes that any increased activity on the acetyl CoA branch, i.e. yeast growth, will increase ester production while other authors [Narziss 2005, Clone] state that increased yeast growth leads to a decrease in esters since more of the acetyl CoA is used for sterol synthesis.
The following is a list of the factors that are known to affect the ester production:
- Yeast stain: the choice of yeast strain has a significant impact on the easter production. But according to Narziss is a yeast strains ability to form esters strongly dependent on the wort composition and high ester levels in one type of wort are not necessarily an indocation that the same strain will also produce high levels of esters in another wort [Hermann 2005].
- availability of oxygen: It has been shown that increased oxygenation rates can lower the amount of esters produced by yeast [Narziss 2005, Noonan 1996, Fix 1999]. This has been explained by the increased production of biomass. When free oxygen is available, the acyl CoA is more likely to be used for the production of sterols and the amount available for ester production will be reduced.
- Double batch (German: Drauflassen): As noted earlier, any process that keeps the yeast in the growth phase decreased ester production. One of these methods is Drauflassen or double batch brewing. A fresh batch of aerated wort is added to the fermenter when the previous batch is at high kraeusen. This can be done multiple times and the continued yeast growth will reduce the amount acyl CoA that can be used for ester formation [Narziss 2005].
- Temperature: Higher fermentation temperatures are known to increase the production of fermentation byproducts including esters. Some authors have explained this with increased yeast metabolism. Saerens et. al. [Saerens 2007] gives this explanation "According to Suomaleinen [Suomalainen 1981], an increase in the fermentation temperature releases higher levels of esters through more efficient excretion and/or enhanced autolysis of the yeast. An effect of the temperature on the thermodynamic equilibrium of ester solubility in cellular lipids and the aqueous medium is another possibly more likely explanation". But it should be noted that the effect of temperature on the ester production is different between yeast strains and also vary between the types of esters that are produced [Hermann 2005]
- pressure: Even though increased CO2 pressure leads to the retardation of yeast growth, it has been shown to reduce the amount of esters (and other fermentation byproducts) that are produced. The increased CO2 pressure is affecting the synthesis of acetyl CoA which results in retarded yeast growth and lower ester production [Hermann 2005]. This shows that if yeast growth is slowed by reduced acetyl CoA levels, the ester production will not be increased.
- pitching rate: Higher yeast pitching rates lead to increased ester levels in the beer [Hermann 2005]. This statement conflicts with common home brewer knowledge [Fix 1999], that underpitching leads to less clean fermentations. According to Narziss, increased ester production is a result of the reduced yeast growth that results from high pitching rates [Narziss 2005].
- FAN levels: It has been shown that an increase in free amino nitrogen (FAN) leads to an increase in ester production [Saerens 2007]. Free amino nitrogen refers to the short length proteins that the yeast absorbs during fermentation. This leads to the conclusion that beers brewed with a high percentage of adjuncts (corn, rice) should show less ester production and that beers with a high FAN level (extended protoelytic activity during the mash. i.e. long protein rest at 50C) should contain more esters.
- wort composition: The higher the percentage of maltose in the wort, the less esters will be produced. Hermann showed that an increase in the worts glucose concentration leads to an increased ester production. The necessary increase in glucose can be achieved through corn-sugar additions or mashing procedures that take advantage of the maltase enzyme. This enzyme can create glucose from maltose, but is not active in typical mashing as it is deactivated above 45 C [Hermann 2005]. Hermann explained that with a delayed occurence of the diauxic point (when the yeast has to switch from glucose to maltose as their primary sugar source). This switch causes the yeast stress under which it produces more esters. If it is delayes, more yeast will encouter it due to the increased yeast growth that has happened up to this point [Hermann 2005].
- wort gravity: The higher the wort gravity, the more esters will be produced during fermentation [Saerens 2007]. The higher wort gravity provides more compounds for enzymatic reactions, the length of the fermentation is increased and the increased sugar concentration inhibits yeast growt which leads to more Acyl-CoA for ester production [Hermann 2005]
From a home brewers point of view only these parameters can be easily varied:
- yeast strain
- oxygenation
- pitching rate
- temperature
- wort composition (through mashing or sugar additions)
The objective of this experiment was to evaluate the qualitative effects of oxygenation and pitching rate on the aroma compounds created by a Weissbier yeast.
Materials and Methods
For this experiment a 12 Plato wort was brewed from a the following grist:
- 70% Weyermann light Wheat
- 30% Weyermann Bohemian Pilsner
A Hochkurz mash with the following rests was used to mash the grain:
- Dough-in, 59 C, 15 min, infusion
- Maltose, 63 C, 45 min, infusion
- Dextrinization, 69 C 20 min, infusion
- Mash-out, 76 C, 10 min, decoction
The 25L preboil volume wort was then boiled for 60 min with 10g Target hops (10% a-acids). The wort was cooled to a pitching temperature of 17C. 9 2 liter sanitized PET Pepsi Cola bottled were filled with 1.5 l each. The wort was then oxygenated using a sintered stainless steel oxygen stone and a regulator. Previously it was determined that the flow rate of oxygen from the oxygen stone at the chosen regulator setting is about 0.6 mg/s. After that the yeast was pitched. The used yeast originated from a pack of Wyeast 3056. I later realized that this is actually a blend and since I have been storing and propagating this yeast for a while I have no idea how the relationship between the yeast strains that were blended changed. The pitched yeast culture came from the slurry of a primary fermentation that started 2 weeks earlier.
The fermentation samples were oxygenated and pitched as follows:
A
1.6 ml/l yeast slurry 1 s oxygen (0.4 ppm) |
B
5.0 ml/l yeast slurry 1 s oxygen (0.4 ppm) |
C
15 ml/l yeast slurry 1 s oxygen (0.4 ppm) |
D
1.6 ml/l yeast slurry 3 s oxygen (1.2 ppm) |
E
5.0 ml/l yeast slurry 3 s oxygen (1.2 ppm) |
F
15 ml/l yeast slurry 3 s oxygen (1.2 ppm) |
G
1.6 ml/l yeast slurry 9 s oxygen (3.6 ppm) |
I
5.0 ml/l yeast slurry 9 s oxygen (3.6 ppm) |
H
15 ml/l yeast slurry 9 s oxygen (3.6 ppm) |
The oxygenation times were taken from my standard ale oxygenation time of 60s for 20l of wort. I later calculated the amount of O2 that was released from the stone, but not all of the released O2 was actually absorbed by the wort. In addition to that the head space also contained oxygen, even more than was added through the oxygen stone. As a result of that the oxygenation part of this experiment cannot be deemed reliable.
The pitching rates amount to the following pitching rates for 20l wort:
- 1.6 ml/l -> 32 ml yeast slurry
- 5 ml/l -> 100 ml yeast slurry
- 15 ml/l -> 300 ml yeast slurry
The actual number of viable cells was not determined
The fast ferment test showed a limit of attenuation of 78% (apparent extract = 2.6 Plato).
All samples were fermented in a single water bath at an average temperature of 19 C (peaked at 20C on day 4). On day 6 the samples were bottled in 12 oz bottles with the addition of corn sugar for carbonation. The amount of corn sugar was based on the current extract level of the beer in order to achieve similar carbonation levels despite the fact that the samples had different extract levels at the time of bottling.
The bottles were then stored at about 19C for 8 more days before they were moved to an ambient temperature of 15 C where they sat before sampling.
Results and Discussion
Fermentation performance
On day 5 the following extract levels were measured for the 9 samples:
With some outliers, the results matches the expectation that higher pitching rates and more oxygen lead to a faster fermentation. The floor in the graph has been set to the extract level that was determined though the fast ferment test (2.6 Plato).
On day 6 (bottling day), the extract levels were not as consistent
While the samples don't seem to follow the expected trend for oxygenation times, they seem to follow the expected trend for the pitching rates. Two of the samples with the highest pitching rate were able to reach the limit of attenuation (78% or AE = 2.6 Plato). The absence of a clear relationship between the oxygenation rate and the fermentation performance is assumed to be a result of the imprecise oxygenation which was discussed earlier.
Taste test
The following are notes on the aroma and taste tests that were done on the various fermentation samples. All taste tests were done by only one taster and none of the beers exhibited a strong ester profile to begin with. This was an indication that the used yeast may not have been the best for this experiment.
12 days after bottling the samples A, B and C (lowest oxygneation, 3 different pitching rates) were evaluated for taste and aroma. It was notes that sample A was less estery than sample C but the difference was very sublte. Actually so subtle that three triangle blind tests between samples A and C, that were done in succession, did not yield a conclusive result. A slight "band aid" taste and aroma was also noted for sample A, but not strong enough for sample A to stand out in the triangle test.
18 days after bottling, the samples G, H and I (highest oxygenation, 3 different pitching rates) were evaluated for taste and aroma. The results were similar to the first taste test and no conclusive difference was found in 3 triangle tests between G and I, though it was noted that H showed slight "band aid" aroma and taste immediately after pouring.
20 days after bottling D, E and F (medium oxygen, 3 different pitching rates) were evaluated. Sample D (lowest pitching rate) showed a band aid aroma and taste, but besides this the aroma and taste between all three samples is very close and it was difficult to tell them apart.
21 days after bottling B and H (medium pitching rate, lowest and highest oxygen) were compared. No "band aid" was found in either sample but B was regarded as less clean and appeared to have more fusel alcohols. But the taste and aroma of both samples was almost the same. No difference in the ester levels was perceived.
31 days after bottling A (lowest oxygen and lowest pitching rate) was compared against I (highest pitching rate and oxygen). Both were remarkably close to each other but I seemed more "rounded" in its taste than A without being able to say exactly why. No significant "band aid" was found in either sample.
Given the fact that none of the performed blind tastings was able to definitively tell a difference between 2 beers, it certainly possible that the little differences that were perceived were influenced by the knowledge of which sample was tasted.
Conclusion
Despite the 9 fold difference in pitching rate and oxygen injection, which showed differences in the fermentation performance, the samples tasted remarkably similar and none of the blind taste tests could tell them apart. The actual difference in dissolved oxygen that was available to the yeast was likely much smaller, but the pitching rates were definitely widely different.
This experiment needs to be seen as inconclusive and further experiments need to be done with a different yeast. But at that point only 3 or 4 fermentations should be run to test either the influences of the pitching rate or the oxygen levels. Having only 3 to 4 samples that need to be evaluated is more manageable than 9 samples.
Sources
- [Clone 1] Danstar FAQ: Yeast Growth
- [Wikipedia] Wikipedia: Ester
- [Hermann 2005] M. Hermann, Entstehung und Beeinflussung qualitätsbestimmender Aromastoffe bei der Herstellung von Weißbier, Dissertation, Technical University Munich, 2005
- [Walsh] A. Walsh, Ester Formation, www.brewery.org
- [Noonan, 1996] Gregory J. Noonan, New Brewing Lager Beer, Brewers Publications, Boulder CO, 1996
- [Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan), Abriss der Bierbrauerei. WILEY-VCH Verlags GmbH Weinheim Germany, 2005
- [Fix, 1999] George J. Fix Ph.D, Principles of Brewing Science, Brewers Publications, Boulder CO, 1999
- [Saerens 2007] S. M. G. Saerens et. al., Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation, www.pubmedcentral.nih.gov
- [Suomalainen 1981] Suomalainen, H. 1981. Yeast esterases and aroma esters in alcoholic beverages. J. Inst. Brew. 87:296-300.