Difference between revisions of "Carbohydrates"
(→Starch) |
|||
| (84 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | {| style="width:800px" | ||
| + | | | ||
| − | + | Carbohydrates are organic molecules that contain carbon, oxygen and hydrogen and are the most abundant organic compounds in nature. They serve as forms of energy source, storage and structural components for plants and some animals [Champe]. They have the basic formula (CH<sub>2</sub>O)<sub>n</sub> hence the name Carbohydrate or hydrate of carbon. Important carbohydrates in brewing are simple sugars (e.g. glucose, fructose), complex sugars (e.g. maltose, sucrose, maltotriose), dextrins and starches. But β-glucans, pectins and gums are also carbohydrates and may also be more or less important in brewing. | |
| − | + | The following sections give insight into the structure of carbohydrates important to brewing. Some are more and others (e.g. lactose and sucrose) are less important in mashing but may be used at other places in the brewing process. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| + | =Monosaccharides= | ||
| − | =References= | + | Monosaccharides are the simplest carbohydrates and have the basic formula C<sub>n</sub>H<sub>2n</sub>O<sub>n</sub>. They are the building blocks of the more complex carbohydrates. The monosaccharides important to brewing, glucose, galactose and fructose, are hexoses which contain 6 carbon atoms. They differ either in their carbonyl group or the orientation of the OH and H groups along the carbon chain. The carbonyl group is the group that contains the oxygen that is linked twice to a carbon atom. Either to the same carbon atom or two different atoms from the same chain. Glucose and galactose are called adelose sugars because their carbonyl group is an aldehyde group (Figures 1 and 3). Fructose however is a ketose sugar and has a Ketone group (Figure 2) as its carbonyl group. |
| + | |||
| + | Monosaccharides may exist in a open chain or in a ring configuration. But for monosaccharides with more than five carbons only less than one percent of the molecules will be in the open chain configuration. The rest is in the ring configuration. The rings are formed by the oxygen atom from the carbonyl group binding to another carbon atom of the chain. | ||
| + | |||
| + | By convetion the carbon atoms are numbered such that the highest numbered carbon is farthest away from the carbonyl (aldehyde or ketone) group. | ||
| + | |||
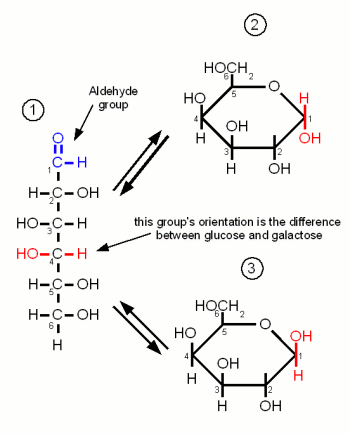
| + | ==Glucose== | ||
| + | |||
| + | [[Image:Glucose.gif|frame|right|Figure 1 - Three configurations of glucose. (1) the open chain form. (2) glucose in the α ring configuration (α-glucopyranose). (3) glucose in the β ring configuration (β-glucopyranose). The ring configuration is drawn both in the Fisher projection (left), which is better in showing how the oxygen atom bridges between carbons 1 and 4, and the more familiar Hanworth projection (right)]] | ||
| + | |||
| + | Glucose is by far the most important monosaccharide in brewing. It is the building block for starch and thus produced during mashing. When the oxygen of the aldehyde group binds between carbon 1 and 5 the molecule forms the ring typical for carbohydrates. The resulting molecule is called glucopyranose and depending on the orientation of the OH group on carbon 1 the molecule may be in the α or the β configuration. The difference is subtle but becomes important later when the glucose binds to other molecules to form more complex sugars. At this point α vs. β configuration becomes permanent and makes the difference between being convertible by mash and yeast enzymes or not. But as long as the glucose molecule is on its own both configurations coexist in an equilibrium and can change between the configurations by changing back to the open chain and back to the ring in the other configuration | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
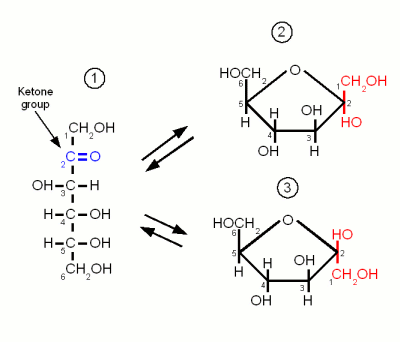
| + | ==Fructose== | ||
| + | [[Image:Fructose.gif|frame|right|Figure 2 - Like glucose, fructose can exist in the open chain form (1) or two ring configurations. α-D-fructofuranose (2) and β-D-fructofuranose (3)]] | ||
| + | |||
| + | Like glucose, fructose is also a hexose sugar but unlike glucose it is a ketose sugar because it has a ketone group as its carbonyl group. This group contains carbon 2 (instead of the carbon 1 that is included in glucose's aldehyde group) and as a result the ring that is formed by the oxygen atom of that group binding to carbon 5 has a pentagon shape in the Hanworth projection (Figure 2). | ||
| + | |||
| + | Fructose has no importance to mashing. It is only present in malt in very small amounts. It may come into beer in substantial amounts through the addition of fruit juices, sucrose or invert sugar. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==Galactose== | ||
| + | |||
| + | [[Image:Galactose.gif|frame|right|Figure 3 - Galactose is very similar to glucose. It is an aldehyde sugar that differs only in the orientation of the hydroxyl (OH) group attached to carbon 4.]] | ||
| + | |||
| + | Galactose is of no importance to mashing as it is neither present in malt nor the produced wort. It's only importance to brewing is through the addition of lactose, which is made of glucose and galactose, as a sweet but unfermentable sugar in the production of certain beer styles (e.g. milk stouts). Like glucose it is an adelose sugar but it differs from glucose in the orientation of the OH group on carbon 4. | ||
| + | |||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | =Disaccharides= | ||
| + | |||
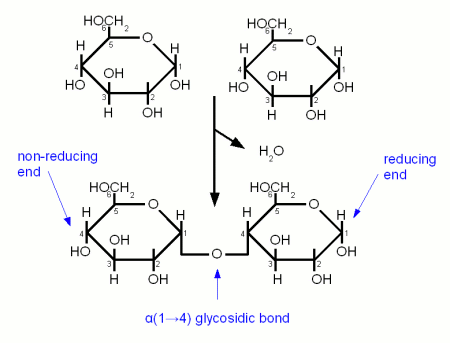
| + | ==The glycosidic bond== | ||
| + | |||
| + | [[Image:Glycosidic_bond.gif|frame|right|Figure 4 - 2 Glucose molecules linking together with an α(1→4) glycosidic bond. This reaction liberates one water molecule per link that is formed. Because only one Glucose's carbonyl group is involved in the link, the resulting disaccharide (maltose) has a reducing and a non-reducing end.]] | ||
| + | |||
| + | A glycosidic bond is formed when the carbonyl group of one monosaccharide reacts with a hydroxyl (OH) group of another molecule and water is eliminated [Vaclavik]. It is the "glue" that holds together monosaccharides to form more complex carbohydrates. When a glycosidic bond is formed the molecule with the carbonyl group that is involved in the bond gets locked into the α or β configuration. Enzyme can distinguish between these two types of bonds and that's why it is important to note the type of a bond. α links hold together fermentable carbohydrates (disaccharides and trisacharides) or carbohydates that can be made fermentable in mashing (dextrins and starches) while β links are the links that hold together structural malt components (cellulose, β-glucans). By convention the carbon atoms that are involved in the bond are listed when the type of gycosidic bond is specified. For example, an α-1,4 (or α(1→4)) glycosidic bond is between carbon 1 (carbonyl group) of one molecule and carbon 4 of the other molecule. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==reducing vs. non-reducing sugars== | ||
| + | |||
| + | If a sugar has a free carbonyl (aldehyde or ketone) group, it is called a reducing sugar. All monosaccharides are reducing sugars as their carbonyl group is not involved in any bond to another molecule. Reducing sugars are able to take part in browning reactions with amino acids called Maillard reactions while non-reducing sugars aren't. But reducing and non-reducing sugars are able to caramelize. | ||
| + | |||
| + | In mashing it is important to distinguish between the reducing and the non-reducing end of a glucose chain as enzymes like beta amylase can only work on the non reducing end. The reducing end is the end with the free carbonyl group (carbon 1 for glucose) and can attach to other glucose chains through a glycosidic bond. | ||
| + | |||
| + | Sucrose for example is a non-reducing sugar as both the aldehyde group of the glucose and the ketone group of the fructose are involved in the α(1→2) link that holds both these molecules together. But sucrose can be converted to the reducing sugars glucose and fructose by making invert sugar through the use of an enzyme (intertase) or through the application of heat in an acidic environment. | ||
| + | |||
| + | Maltose and lactose are examples for disaccharides that are reducing sugars. | ||
| + | |||
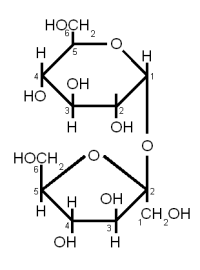
| + | ==Maltose and Cellobiose== | ||
| + | |||
| + | [[Image:Cellobiose.gif|frame|right|Figure 5 - Both maltose and cellobiose are composed of two glucose molecules. But in maltose the glucose molecules are in the α configuration and joined with an α(1→4) link while in cellobiose they are in the β configuration and joined with a β(1→4). The result is that maltose can be metabolized by yeast while cellobiose cannot.]] | ||
| + | |||
| + | Both Maltose and Cellobiose consist of 2 glucose molecules linked. In maltose they are liked with an α(1→4) link while in Cellibiose they are linked with a β(1→4) link. The result is that maltose can be fermented by yeast while cellibose cannot. | ||
| + | |||
| + | Maltose is the most abundant sugar in wort and hence very important in brewing. It is created by the enzymatic hydrolyzation (breakdown) of starch. Cellobiose is the building block of cellulose (β-glucans) and has some importance in brewing. Mainly with respect to lauter problems caused by excessive amounts of β-glucans in the wort. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==Sucrose== | ||
| + | |||
| + | [[Image:Sucrose.gif|frame|right|Figure 6 - Sucrose is formed by an α(1→2) link between glucose and fructose. Note that the carbonyl groups of both sugars are involved in the bond. As a result sucrose is not a reducing sugar]] | ||
| + | |||
| + | Sucrose is a disaccharide consisting of glucose and fructose connected together with an α(1→2) bond (Figure 6). Sucrose is also known as white or table sugar and is produced from sugar beets or sugar cane. While it is present in malt in small amounts (~5% of carbohydates) it is only affected little by mashing and survives into the sweet wort. More substantial amounts of sucrose may be added as kettle adjunct or when the beer is primed with table sugar. Sucrose is a non reducing sugar which means it won't take part in Maillard reactions. | ||
| + | |||
| + | ===Invert sugar=== | ||
| + | |||
| + | Invert sugar is an equal mix of glucose and fructose and is made from sucrose by breaking the glycosidic bond through enzymes (invertase) or the application of heat in an acidic environment. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==Lactose== | ||
| + | |||
| + | [[Image:Lactose.gif|frame|right|Figure 7 - Lactose]] | ||
| + | |||
| + | Lactose, also known as milk sugar, cannot be metabolized by brewing yeast and is used in some styles to add sweetness to the finished beer. It is neither present in malt nor a result of mashing and has to be added in the kettle. | ||
| + | |||
| + | Lactose consists of galactose and glucose connected linked together with a β(1→4) link. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==Raffinose and Melibiose== | ||
| + | |||
| + | [[Image:Raffinose.gif|frame|right|Figure 8 – (1) raffinose consists of glactose, glucose and fructose (from the top). The enzyme invertase can split the bond between glucose and fructose to create (2) melibiose and (3) fructose. Melibiose can only be fermented by lager yeasts, but both ale and lager yeasts have the enzyme invertase which enables them to split raffinose]] | ||
| + | |||
| + | While raffinose is not a disaccharide it should be covered here together with melibiose. | ||
| + | |||
| + | Raffinose is absent from brewing wort and melibiose is only present in very small amounts but both sugars are frequently mentioned in connection with the genetic difference between ale yeast (s. cerevisiae) and lager yeast (s. pastorianus). | ||
| + | |||
| + | Melibiose is a disaccharide that is formed by galactose and glucose linked together with a β(1→6) glycosidic bond. Raffinose is a trisaccharide that is formed by attaching a fructose molecule to melibiose’s glucose molecule with an α(1→2) bond. Lager yeasts can completely ferment melibiose and raffinose while ale yeas can only ferment a third of raffinose and no melibiose. Both yeasts have the enzyme invertase between their cell wall and cell membrane. This enzyme breaks the α(1→2) bond of raffinose and creates melibiose and fructose just as it splits the same link in sucrose to create glucose and fructose. The fructose molecule can be transferred into the cell and metabolized by both ale and lager yeasts but only lager yeasts produce the necessary enzyme (α-galactosidase) to break the melibiose into galactose and glucose which can then be metabolized. So when it is said that ale yeast can only ferment one third of raffinose it means that it can only ferment one third of each raffinose molecule and not one third of the amount of raffinose molecules. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | =Oligosaccraides= | ||
| + | |||
| + | [[Image:Oligosaccharides.gif|frame|right|Figure 8 - various oligosaccharides. (1) maltotriose, (2) matltotetralose, (3) limit dextrin. This is a branch point from an amylopectin molecule that cannot be hydrolyzed (i.e. split) by either alpha or beta amylase]] | ||
| + | |||
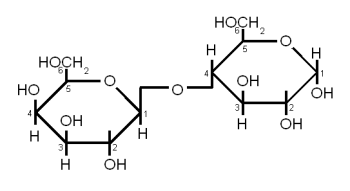
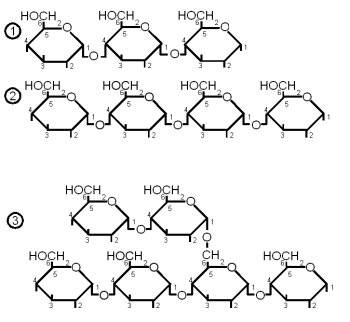
| + | When more than 2 but less than about 10 monosaccharides are linked together the resulting sugar is called an oligosaccharide (in Greek ''oligos'' means few). In brewing only glucose based oligosaccharides are of significance. They are fragments of starch chains and depending on their length may or may not be metabolized by yeast. | ||
| + | |||
| + | * maltotriose: 3 glucose molecules linked together with an α(1→4) bond. This sugar can be fermented by both ale and lager yeast | ||
| + | * maltotetralose: 4 glucose molecules linked with an α(1→4) bond. This sugar cannot be fermented by ale or lager yeast | ||
| + | * maltopentalose: 5 glucose molecules linked with an α(1→4) bond. This sugar cannot be fermented by ale or lager yeast | ||
| + | * linear dextrins: 6 or more glucose molecules linked with α(1→4) bonds. Not fermentable by ale or lager yeast | ||
| + | * limit dextrins: small branched glucose chains. The branch point is created by an α(1→6) bond. These dextrins stem from the branch points in amylopectin and cannot be hydrolyzed by α or β-amylase. The enzyme that can break them is limit dextrinase, but that enzyme is quickly denatured above 60 C (140F). But before that temperature is reached only little of the starch has be gelatinized (at least in infusion mashing) which limits the enzyme's access to branch points to work on. More information about the process of starch conversion and the enzymes that are involved in it is given in [[Starch Conversion]]. This dextrin cannot be fermented by either ale or lager yeast. | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | ==Reaction with iodine== | ||
| + | |||
| + | [[Image:Iodine_test.jpg|frame|right|Figure 9 - reaction between iodine and mash liquid on chalk. (A) lots of starch and large dextrins present, (B) large dextrins (branched and unbranched) present, (C) iodine-negative mash. At this point no or very little large dextrines are present]] | ||
| + | |||
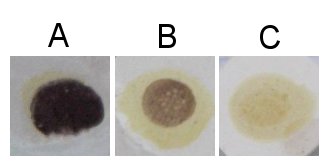
| + | When the glucose chains are long enough (more than about 9 glucose molecules for unbranched chains) the glucose chain (i.e. dextrine) forms a helical structure that is supported by weak bonds between the hydroxyl (OH) groups of the glucose molecules. This structure can absorb one or more triiodide (I<sub>3</sub><sup>-</sup>) ions which results in a color reaction. This is the chemistry behind the iodine test that us used to detect the presence of starch or large dextrines. Iodine is poorly soluble in water but it can be made soluble through the presence of potassium iodine (KI) the result is triiodide (I<sub>3</sub><sup>-</sup>) which can react with the glucose chains in the aforementioned reaction [Elmhurst]. | ||
| + | |||
| + | The resulting color depends on the length of the glucose chains. Shorter chains (starting at about 9 glucose molecules in unbranched chains and up to 60 glucose molecules in branches chains) give a red color [Narziss, 2005]. These dextrines are also called erythrodextrines [Kunze, 2007]. Amylose, which consists of very long glucose chains between occasional branch points and very large dextrines gives a dark blue color while amylopectin, which has much more branch points and shorter glucose chains between these branch points, gives a more reddish color in the presence of iodine. | ||
| + | |||
| + | Brewing wort should show no visible reaction with iodine, This means that no glucose chains longer than about 9 glucose molecules are present. Otherwise the beer can suffer from a starch haze that is difficult to remove. This haze is actually not caused by starch but by large dextrines which become less soluble as the alcohol content increases during fermentation [Narziss, 2005]. | ||
| + | |||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | =Starch= | ||
| + | |||
| + | Starch is a mixture of 2 polymers of glucose: amylose and amylopectin. The glucose molecules are linked in their α configuration, which makes them α-glucans. Through photosynthesis plants produce glucose which, as sucrose, can be transported to the roots (e.g. potato) or seeds (e.g. barley) where it is used to create amylose and amylopectin that is then stored in starch granules. While crops exist that produce amylose free starched (called waxy starches) most plant starch (including barley) consists of 20 - 26% of amylose and the rest being amylopectin. | ||
| + | |||
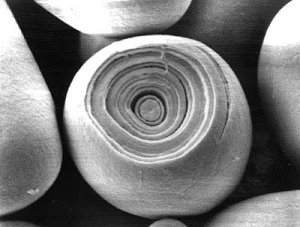
| + | [[Image:starch_kernel.jpg|right|frame|Figure 10 - crossection of a starch kernel under an electron microscope [foodnews.ch]]] | ||
| + | |||
| + | Amylose is a polymer of glucose that contains on average 1600-1900 glucose molecules that are held together by α(1→4) bonds (Figure 11.g). But occasional α(1→6) branch points exists which has been shown by the incomplete hydrolysis of amylose by β-amylase [Briggs, 2004]. With iodine amylose gives a characteristic black-blue color. | ||
| + | |||
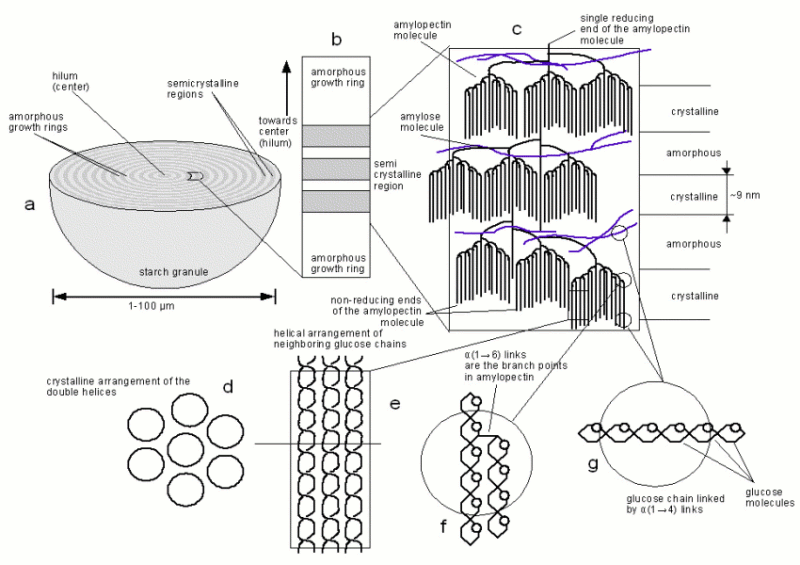
| + | Amylopectin contains on average 10-20 times more glucose molecules than amylose. It is highly branched and the branch points, α(1→6) links (Figure 11.f), account for 6-7% of all bonds [Kunze, 2007]. The rest of the bonds are α(1→4) links. Due to the shorter length of the α(1→4) linked sections amylopectin gives a more reddish color in the presence if iodine. It's structure is shown in Figure 11.c. The glucose chain that contains the only reducing end, which within the starch granule is oriented towards the center or hilum of the granule, is called the C chain. Attached to it with α(1→6) links are B chains. B chains can support other B chains or A chains. The A chains are the outermost chains which don't support any other chains. A and B chains forms clusters and B chains can span and support multiple clusters. A chains typically consist of 6-15 glucose molecules while B chains may contain from 15-25 up to 50 and more glucose molecules depending on how many clusters they span [Donald, 2004]. In the section which doesn't contain α(1→6) branch points two neighboring glucose chains will form a double helix (Figure 11.e) and these double helices then arrange in a crystalline pattern. Figure 11.d shows that pattern when their cross section is viewed. All these structures are held together by hydrogen bonds and will break when the thermal energy of the water is large enough. This process is called gelatinization and will be explained later. | ||
| + | |||
| + | The sections where the branch points of the amylopectin are located are not crystalline, they are amorphous and may also contain amylose molecules. Yet another but larger amorphous regions (Figure 11.b) are the growth rings which can be seen under the microscope when a starch granule is cut open (Figure 10). The origin of these growth rings is not entirely clear but it has been shown that they may stem from fluctuations during the growth of the starch granule. For wheat and barley it has been shown that their starch granules will not show growth rings if the plant is grown under constant environmental conditions [Donald, 2004] | ||
| + | |||
| + | <div style="clear:both;"></div> | ||
| + | [[Image:Starch.gif|frame|center|Figure 11 - The structure of a starch granule at different length scales]] | ||
| + | <div style="clear:both;"></div> | ||
| + | |||
| + | The shape of the starch granule can range from spherical to elliptical and lenticular while some (e.g. rice) can also exhibit sharp edges. When viewed under crossed polarized light the starch granules show a characteristic Maltese cross which confirms the presence of ordered crystalline structures [Donald, 2004]. | ||
| + | |||
| + | In Barley, two different size groups of starch granules exist. The large A granules have diameters between 22 and 48 μm and make up 10-20% by number but 85-90% by weight. The balance are the smaller B granules which diameters of only 1.7-2.5 μm [Briggs, 2004]. Starch is often infested with protein which may hinder its breakdown. This is not a problem in well modified malts where the protein degradation has been driven far enough but in undermodified malts. These malts show an increased conversion efficiency when a sufficient protoelytic activity is provided through a protein rest. | ||
| + | |||
| + | =References= | ||
| + | |||
| + | :[Valclavik] Vickie A. Valclavik, Elizabeth W. Christian, ''Essentials of Food Science'', Third Edition, Springer | ||
| + | :[Champe] Pamela C, Champe, Richard A. Harvey, Denise R. Ferrier, ''Biochemistry'', Lippincott's Illustrated Reviews | ||
| + | :[Elmhurst] [http://www.elmhurst.edu/~chm/vchembook/548starchiodine.html Starch - Iodine], Elmhurst College | ||
| + | :[Kunze, 2007] Wolfgang Kunze, ''Technologie Brauer und Maelzer'', 9. Auflage, VLB Berlin | ||
| + | :[Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, ''Abriss der Bierbrauerei'', Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan). WILEY-VCH Verlags GmbH Weinheim Germany, 2005 | ||
| + | :[foodnews.ch] [http://www.foodnews.ch/x-plainmefood/20_lebensmittel/Staerke.html ''Saerke''] [http://www.foodnews.ch/x-plainmefood/index.html www.foodnews.ch] | ||
| + | :[Donald, 2004] A. M. Donald, ''Understanding Starch Stucture and Functionality'', Chapter 5 in ''Starch in Food: Structure, Function and Applications'' By Ann-Charlotte Eliasson, CRC Press, 2004 | ||
| + | |||
| + | |} | ||
Latest revision as of 03:06, 15 April 2009
|
Carbohydrates are organic molecules that contain carbon, oxygen and hydrogen and are the most abundant organic compounds in nature. They serve as forms of energy source, storage and structural components for plants and some animals [Champe]. They have the basic formula (CH2O)n hence the name Carbohydrate or hydrate of carbon. Important carbohydrates in brewing are simple sugars (e.g. glucose, fructose), complex sugars (e.g. maltose, sucrose, maltotriose), dextrins and starches. But β-glucans, pectins and gums are also carbohydrates and may also be more or less important in brewing. The following sections give insight into the structure of carbohydrates important to brewing. Some are more and others (e.g. lactose and sucrose) are less important in mashing but may be used at other places in the brewing process. Contents[hide]MonosaccharidesMonosaccharides are the simplest carbohydrates and have the basic formula CnH2nOn. They are the building blocks of the more complex carbohydrates. The monosaccharides important to brewing, glucose, galactose and fructose, are hexoses which contain 6 carbon atoms. They differ either in their carbonyl group or the orientation of the OH and H groups along the carbon chain. The carbonyl group is the group that contains the oxygen that is linked twice to a carbon atom. Either to the same carbon atom or two different atoms from the same chain. Glucose and galactose are called adelose sugars because their carbonyl group is an aldehyde group (Figures 1 and 3). Fructose however is a ketose sugar and has a Ketone group (Figure 2) as its carbonyl group. Monosaccharides may exist in a open chain or in a ring configuration. But for monosaccharides with more than five carbons only less than one percent of the molecules will be in the open chain configuration. The rest is in the ring configuration. The rings are formed by the oxygen atom from the carbonyl group binding to another carbon atom of the chain. By convetion the carbon atoms are numbered such that the highest numbered carbon is farthest away from the carbonyl (aldehyde or ketone) group. Glucose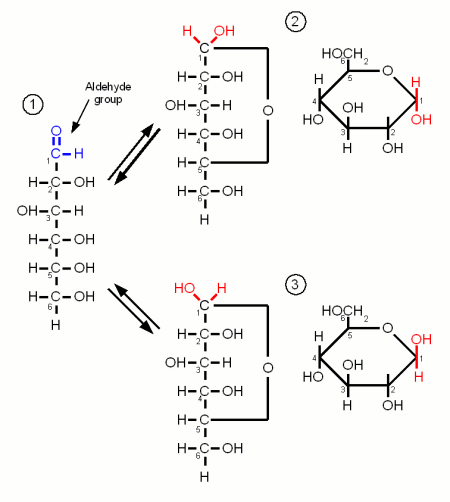 Figure 1 - Three configurations of glucose. (1) the open chain form. (2) glucose in the α ring configuration (α-glucopyranose). (3) glucose in the β ring configuration (β-glucopyranose). The ring configuration is drawn both in the Fisher projection (left), which is better in showing how the oxygen atom bridges between carbons 1 and 4, and the more familiar Hanworth projection (right) Glucose is by far the most important monosaccharide in brewing. It is the building block for starch and thus produced during mashing. When the oxygen of the aldehyde group binds between carbon 1 and 5 the molecule forms the ring typical for carbohydrates. The resulting molecule is called glucopyranose and depending on the orientation of the OH group on carbon 1 the molecule may be in the α or the β configuration. The difference is subtle but becomes important later when the glucose binds to other molecules to form more complex sugars. At this point α vs. β configuration becomes permanent and makes the difference between being convertible by mash and yeast enzymes or not. But as long as the glucose molecule is on its own both configurations coexist in an equilibrium and can change between the configurations by changing back to the open chain and back to the ring in the other configuration FructoseLike glucose, fructose is also a hexose sugar but unlike glucose it is a ketose sugar because it has a ketone group as its carbonyl group. This group contains carbon 2 (instead of the carbon 1 that is included in glucose's aldehyde group) and as a result the ring that is formed by the oxygen atom of that group binding to carbon 5 has a pentagon shape in the Hanworth projection (Figure 2). Fructose has no importance to mashing. It is only present in malt in very small amounts. It may come into beer in substantial amounts through the addition of fruit juices, sucrose or invert sugar. GalactoseGalactose is of no importance to mashing as it is neither present in malt nor the produced wort. It's only importance to brewing is through the addition of lactose, which is made of glucose and galactose, as a sweet but unfermentable sugar in the production of certain beer styles (e.g. milk stouts). Like glucose it is an adelose sugar but it differs from glucose in the orientation of the OH group on carbon 4.
DisaccharidesThe glycosidic bondA glycosidic bond is formed when the carbonyl group of one monosaccharide reacts with a hydroxyl (OH) group of another molecule and water is eliminated [Vaclavik]. It is the "glue" that holds together monosaccharides to form more complex carbohydrates. When a glycosidic bond is formed the molecule with the carbonyl group that is involved in the bond gets locked into the α or β configuration. Enzyme can distinguish between these two types of bonds and that's why it is important to note the type of a bond. α links hold together fermentable carbohydrates (disaccharides and trisacharides) or carbohydates that can be made fermentable in mashing (dextrins and starches) while β links are the links that hold together structural malt components (cellulose, β-glucans). By convention the carbon atoms that are involved in the bond are listed when the type of gycosidic bond is specified. For example, an α-1,4 (or α(1→4)) glycosidic bond is between carbon 1 (carbonyl group) of one molecule and carbon 4 of the other molecule. reducing vs. non-reducing sugarsIf a sugar has a free carbonyl (aldehyde or ketone) group, it is called a reducing sugar. All monosaccharides are reducing sugars as their carbonyl group is not involved in any bond to another molecule. Reducing sugars are able to take part in browning reactions with amino acids called Maillard reactions while non-reducing sugars aren't. But reducing and non-reducing sugars are able to caramelize. In mashing it is important to distinguish between the reducing and the non-reducing end of a glucose chain as enzymes like beta amylase can only work on the non reducing end. The reducing end is the end with the free carbonyl group (carbon 1 for glucose) and can attach to other glucose chains through a glycosidic bond. Sucrose for example is a non-reducing sugar as both the aldehyde group of the glucose and the ketone group of the fructose are involved in the α(1→2) link that holds both these molecules together. But sucrose can be converted to the reducing sugars glucose and fructose by making invert sugar through the use of an enzyme (intertase) or through the application of heat in an acidic environment. Maltose and lactose are examples for disaccharides that are reducing sugars. Maltose and Cellobiose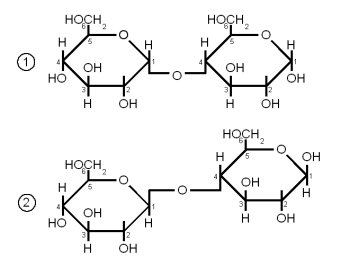 Figure 5 - Both maltose and cellobiose are composed of two glucose molecules. But in maltose the glucose molecules are in the α configuration and joined with an α(1→4) link while in cellobiose they are in the β configuration and joined with a β(1→4). The result is that maltose can be metabolized by yeast while cellobiose cannot. Both Maltose and Cellobiose consist of 2 glucose molecules linked. In maltose they are liked with an α(1→4) link while in Cellibiose they are linked with a β(1→4) link. The result is that maltose can be fermented by yeast while cellibose cannot. Maltose is the most abundant sugar in wort and hence very important in brewing. It is created by the enzymatic hydrolyzation (breakdown) of starch. Cellobiose is the building block of cellulose (β-glucans) and has some importance in brewing. Mainly with respect to lauter problems caused by excessive amounts of β-glucans in the wort. SucroseSucrose is a disaccharide consisting of glucose and fructose connected together with an α(1→2) bond (Figure 6). Sucrose is also known as white or table sugar and is produced from sugar beets or sugar cane. While it is present in malt in small amounts (~5% of carbohydates) it is only affected little by mashing and survives into the sweet wort. More substantial amounts of sucrose may be added as kettle adjunct or when the beer is primed with table sugar. Sucrose is a non reducing sugar which means it won't take part in Maillard reactions. Invert sugarInvert sugar is an equal mix of glucose and fructose and is made from sucrose by breaking the glycosidic bond through enzymes (invertase) or the application of heat in an acidic environment. LactoseLactose, also known as milk sugar, cannot be metabolized by brewing yeast and is used in some styles to add sweetness to the finished beer. It is neither present in malt nor a result of mashing and has to be added in the kettle. Lactose consists of galactose and glucose connected linked together with a β(1→4) link. Raffinose and Melibiose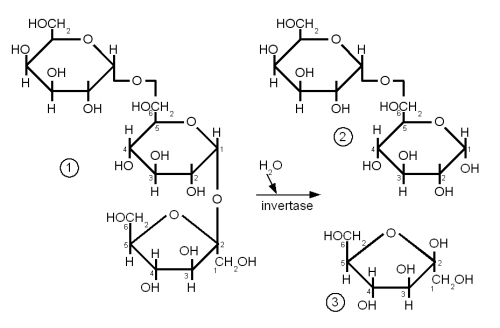 Figure 8 – (1) raffinose consists of glactose, glucose and fructose (from the top). The enzyme invertase can split the bond between glucose and fructose to create (2) melibiose and (3) fructose. Melibiose can only be fermented by lager yeasts, but both ale and lager yeasts have the enzyme invertase which enables them to split raffinose While raffinose is not a disaccharide it should be covered here together with melibiose. Raffinose is absent from brewing wort and melibiose is only present in very small amounts but both sugars are frequently mentioned in connection with the genetic difference between ale yeast (s. cerevisiae) and lager yeast (s. pastorianus). Melibiose is a disaccharide that is formed by galactose and glucose linked together with a β(1→6) glycosidic bond. Raffinose is a trisaccharide that is formed by attaching a fructose molecule to melibiose’s glucose molecule with an α(1→2) bond. Lager yeasts can completely ferment melibiose and raffinose while ale yeas can only ferment a third of raffinose and no melibiose. Both yeasts have the enzyme invertase between their cell wall and cell membrane. This enzyme breaks the α(1→2) bond of raffinose and creates melibiose and fructose just as it splits the same link in sucrose to create glucose and fructose. The fructose molecule can be transferred into the cell and metabolized by both ale and lager yeasts but only lager yeasts produce the necessary enzyme (α-galactosidase) to break the melibiose into galactose and glucose which can then be metabolized. So when it is said that ale yeast can only ferment one third of raffinose it means that it can only ferment one third of each raffinose molecule and not one third of the amount of raffinose molecules. OligosaccraidesWhen more than 2 but less than about 10 monosaccharides are linked together the resulting sugar is called an oligosaccharide (in Greek oligos means few). In brewing only glucose based oligosaccharides are of significance. They are fragments of starch chains and depending on their length may or may not be metabolized by yeast.
Reaction with iodineWhen the glucose chains are long enough (more than about 9 glucose molecules for unbranched chains) the glucose chain (i.e. dextrine) forms a helical structure that is supported by weak bonds between the hydroxyl (OH) groups of the glucose molecules. This structure can absorb one or more triiodide (I3-) ions which results in a color reaction. This is the chemistry behind the iodine test that us used to detect the presence of starch or large dextrines. Iodine is poorly soluble in water but it can be made soluble through the presence of potassium iodine (KI) the result is triiodide (I3-) which can react with the glucose chains in the aforementioned reaction [Elmhurst]. The resulting color depends on the length of the glucose chains. Shorter chains (starting at about 9 glucose molecules in unbranched chains and up to 60 glucose molecules in branches chains) give a red color [Narziss, 2005]. These dextrines are also called erythrodextrines [Kunze, 2007]. Amylose, which consists of very long glucose chains between occasional branch points and very large dextrines gives a dark blue color while amylopectin, which has much more branch points and shorter glucose chains between these branch points, gives a more reddish color in the presence of iodine. Brewing wort should show no visible reaction with iodine, This means that no glucose chains longer than about 9 glucose molecules are present. Otherwise the beer can suffer from a starch haze that is difficult to remove. This haze is actually not caused by starch but by large dextrines which become less soluble as the alcohol content increases during fermentation [Narziss, 2005].
StarchStarch is a mixture of 2 polymers of glucose: amylose and amylopectin. The glucose molecules are linked in their α configuration, which makes them α-glucans. Through photosynthesis plants produce glucose which, as sucrose, can be transported to the roots (e.g. potato) or seeds (e.g. barley) where it is used to create amylose and amylopectin that is then stored in starch granules. While crops exist that produce amylose free starched (called waxy starches) most plant starch (including barley) consists of 20 - 26% of amylose and the rest being amylopectin. Amylose is a polymer of glucose that contains on average 1600-1900 glucose molecules that are held together by α(1→4) bonds (Figure 11.g). But occasional α(1→6) branch points exists which has been shown by the incomplete hydrolysis of amylose by β-amylase [Briggs, 2004]. With iodine amylose gives a characteristic black-blue color. Amylopectin contains on average 10-20 times more glucose molecules than amylose. It is highly branched and the branch points, α(1→6) links (Figure 11.f), account for 6-7% of all bonds [Kunze, 2007]. The rest of the bonds are α(1→4) links. Due to the shorter length of the α(1→4) linked sections amylopectin gives a more reddish color in the presence if iodine. It's structure is shown in Figure 11.c. The glucose chain that contains the only reducing end, which within the starch granule is oriented towards the center or hilum of the granule, is called the C chain. Attached to it with α(1→6) links are B chains. B chains can support other B chains or A chains. The A chains are the outermost chains which don't support any other chains. A and B chains forms clusters and B chains can span and support multiple clusters. A chains typically consist of 6-15 glucose molecules while B chains may contain from 15-25 up to 50 and more glucose molecules depending on how many clusters they span [Donald, 2004]. In the section which doesn't contain α(1→6) branch points two neighboring glucose chains will form a double helix (Figure 11.e) and these double helices then arrange in a crystalline pattern. Figure 11.d shows that pattern when their cross section is viewed. All these structures are held together by hydrogen bonds and will break when the thermal energy of the water is large enough. This process is called gelatinization and will be explained later. The sections where the branch points of the amylopectin are located are not crystalline, they are amorphous and may also contain amylose molecules. Yet another but larger amorphous regions (Figure 11.b) are the growth rings which can be seen under the microscope when a starch granule is cut open (Figure 10). The origin of these growth rings is not entirely clear but it has been shown that they may stem from fluctuations during the growth of the starch granule. For wheat and barley it has been shown that their starch granules will not show growth rings if the plant is grown under constant environmental conditions [Donald, 2004] The shape of the starch granule can range from spherical to elliptical and lenticular while some (e.g. rice) can also exhibit sharp edges. When viewed under crossed polarized light the starch granules show a characteristic Maltese cross which confirms the presence of ordered crystalline structures [Donald, 2004]. In Barley, two different size groups of starch granules exist. The large A granules have diameters between 22 and 48 μm and make up 10-20% by number but 85-90% by weight. The balance are the smaller B granules which diameters of only 1.7-2.5 μm [Briggs, 2004]. Starch is often infested with protein which may hinder its breakdown. This is not a problem in well modified malts where the protein degradation has been driven far enough but in undermodified malts. These malts show an increased conversion efficiency when a sufficient protoelytic activity is provided through a protein rest. References
|