Difference between revisions of "Starch Conversion"
(→pH and brewing water) |
(→pH and brewing water) |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {| style="width:800px" | |
| + | | | ||
90-92% of the solids in brewing wort are carbohydrates [Briggs, 2004]. Proteins make up only 4-5% and the rest are vitamins, minerals and trace elements. Of the carbohydrates more than 95% are products of the starch conversion that happens in the mash tun. As a result the starch degradation is the main purpose of mashing. It produces the fuel that the yeast needs for fermentation from the insoluble starch found in malt and mash tun adjuncts. The extend to which this conversion happens will determine the efficiency with which the ingredients are utilized and the fermentability of the produced wort as well as other quality characteristics. | 90-92% of the solids in brewing wort are carbohydrates [Briggs, 2004]. Proteins make up only 4-5% and the rest are vitamins, minerals and trace elements. Of the carbohydrates more than 95% are products of the starch conversion that happens in the mash tun. As a result the starch degradation is the main purpose of mashing. It produces the fuel that the yeast needs for fermentation from the insoluble starch found in malt and mash tun adjuncts. The extend to which this conversion happens will determine the efficiency with which the ingredients are utilized and the fermentability of the produced wort as well as other quality characteristics. | ||
| Line 7: | Line 8: | ||
=Gelatinization= | =Gelatinization= | ||
| − | Starch granules are insoluble in cold water and will absorb only little water. | + | Starch granules are insoluble in cold water and will absorb only little water. They form a suspension that quickly settles once agitation stops. As the water is heated (50+ C) more and more water is absorbed and the granules start to swell [Narziss, 2005]. The water absorbed during this process can be up to 30 times the weight of the starch granule. This uptake of water initially happens within the amorphous growth rings. At this point the granule starts to leach amylose and the crystalline layers break open and separate from the starch granule as gelatinous sheets. At this point the crystalline structure is lost and the process becomes irreversible with respect to the shape of the starch granule [Shetty, 2006]. The starch granule has gelatinized. |
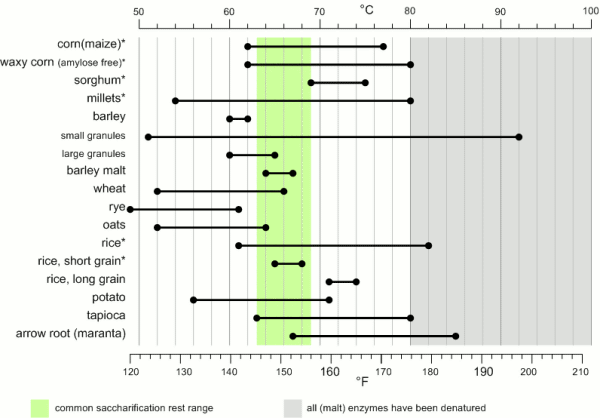
[[Image:Gelatinization_temperatures.gif|frame|right|Figure 1 - Temperature ranges for the gelatinization of various starches [Briggs, 2004]. Starches marked with (*) also benefit from boiling before being used in the mash.]] | [[Image:Gelatinization_temperatures.gif|frame|right|Figure 1 - Temperature ranges for the gelatinization of various starches [Briggs, 2004]. Starches marked with (*) also benefit from boiling before being used in the mash.]] | ||
| Line 32: | Line 33: | ||
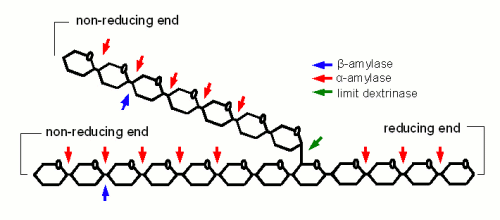
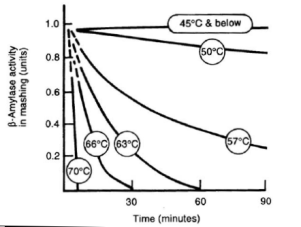
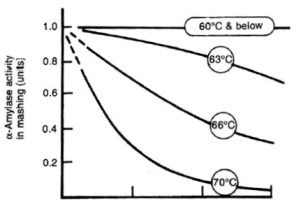
α-amylase is an enzyme that can cleave any α(1-4) bond in starch and dextrines except for the ones close to the branch points. This breakage of a glycosidic bond is also called hydrolyzation as it consumes one water molecule. It is the enzyme that is responsible for the rapid loss of viscosity and iodine reaction after malt starch gelatinized. A process that is called liquefaction of the mash. While α-amylase is very good at breaking down starch into smaller dextrines, which quickly reduces the iodine reaction from a black-blue to a more reddisch brown color, it is not very good at producing fermentable sugars. This results from the low affinity that α-amylase has towards smaller glucose chains. | α-amylase is an enzyme that can cleave any α(1-4) bond in starch and dextrines except for the ones close to the branch points. This breakage of a glycosidic bond is also called hydrolyzation as it consumes one water molecule. It is the enzyme that is responsible for the rapid loss of viscosity and iodine reaction after malt starch gelatinized. A process that is called liquefaction of the mash. While α-amylase is very good at breaking down starch into smaller dextrines, which quickly reduces the iodine reaction from a black-blue to a more reddisch brown color, it is not very good at producing fermentable sugars. This results from the low affinity that α-amylase has towards smaller glucose chains. | ||
| + | |||
| + | {| align="right" border="1" | ||
| + | |+Table 1 - A comparison of the malt carbohydrates and the wort carbohydrates. (Data by Hall ''et. al.'' via [Briggs, 2004]) | ||
| + | |- | ||
| + | ! carbonydrate !! Malt (% of total wort solids) !! Wort (% of total wort solids) | ||
| + | |- | ||
| + | | Starch || 85.8 || 0 || | ||
| + | |- | ||
| + | | Dextrins, Glucans and Pentosans || 2.5 || 22.2 | ||
| + | |- | ||
| + | | Fructans || 1.4 || ? | ||
| + | |- | ||
| + | | Maltotetraose || - || 6.1 | ||
| + | |- | ||
| + | | Maltotriose || 0.6 || 14.0 | ||
| + | |- | ||
| + | | Maltose || 1.0 || 41.1 | ||
| + | |- | ||
| + | | Sucrose || 5.1 || 5.5 | ||
| + | |- | ||
| + | | Glucose + Fructose || 2.4 || 8.9 | ||
| + | |- | ||
| + | | Total || 98.8 || 97.8 | ||
| + | |- | ||
| + | |} | ||
| + | |||
β-amylase is much better at producing fermentable sugars. In fact the way in which β-amylase hydrolyzes starches and large dextrines is the reason for the large maltose content in wort produced through mashing. It attaches to the non-reducing end of a glucose chain and clips off one maltose molecule after another. But because it has to do that at the non-reducing end its activity has to stop when it comes close to a branch point (α(1-6) link). In order to work on the glucose chains after the branch points it relies on the activity of the α-amylase which creates a non-reducing end every time it splits a glucose chain or the limit dextrinase which can break the branching α(1-6) link itself. In mashing α- and β-amylase work in concert: α-amylase creates substrate (i.e. non-reducing ends) for β-amylase. The process of creating sugars is called saccharification and happens mostly after the liquification of the mash. Mashes that intend to produce a highly fermentable wort always try to maximize the β-amylase activity. This is mainly done through the rest temperature(s) and the time spent at these rest(s) which will be discussed later | β-amylase is much better at producing fermentable sugars. In fact the way in which β-amylase hydrolyzes starches and large dextrines is the reason for the large maltose content in wort produced through mashing. It attaches to the non-reducing end of a glucose chain and clips off one maltose molecule after another. But because it has to do that at the non-reducing end its activity has to stop when it comes close to a branch point (α(1-6) link). In order to work on the glucose chains after the branch points it relies on the activity of the α-amylase which creates a non-reducing end every time it splits a glucose chain or the limit dextrinase which can break the branching α(1-6) link itself. In mashing α- and β-amylase work in concert: α-amylase creates substrate (i.e. non-reducing ends) for β-amylase. The process of creating sugars is called saccharification and happens mostly after the liquification of the mash. Mashes that intend to produce a highly fermentable wort always try to maximize the β-amylase activity. This is mainly done through the rest temperature(s) and the time spent at these rest(s) which will be discussed later | ||
| Line 38: | Line 65: | ||
Maltase is an enzyme that has its optimum between 30 and 40 C and can split a single glucose molecule from the non-reducing end of a glucose chain (similar to β-amylase which splits a glucose pair from the non-reducing end of glucose chains). It's affinity to the substrate increases as the degree of polymerization decreases and it is highest for maltose (degree of polymerization is 2) [Kessler, 2006]. But it is generally of little interest in mashing as at its working temperature there is not much maltose present in the wort (which assumes that the mash is doughed in at or below 40 C). If activity of this enzyme is desired to increase the glucose level of the wort the mash needs to be held for saccharification at 63-65C and after having been cooled to 40 C fresh malt is added which also adds new maltase enzymes. After a rest of 30-45 min the mash is heated again to convert the starch that has been added with the new malt. This mash schedule has been introduced by Markus Hermann from the Weihenstephan brewing school to produce high glucose worts for ester rich Weissbiers [Hermann, 2005] | Maltase is an enzyme that has its optimum between 30 and 40 C and can split a single glucose molecule from the non-reducing end of a glucose chain (similar to β-amylase which splits a glucose pair from the non-reducing end of glucose chains). It's affinity to the substrate increases as the degree of polymerization decreases and it is highest for maltose (degree of polymerization is 2) [Kessler, 2006]. But it is generally of little interest in mashing as at its working temperature there is not much maltose present in the wort (which assumes that the mash is doughed in at or below 40 C). If activity of this enzyme is desired to increase the glucose level of the wort the mash needs to be held for saccharification at 63-65C and after having been cooled to 40 C fresh malt is added which also adds new maltase enzymes. After a rest of 30-45 min the mash is heated again to convert the starch that has been added with the new malt. This mash schedule has been introduced by Markus Hermann from the Weihenstephan brewing school to produce high glucose worts for ester rich Weissbiers [Hermann, 2005] | ||
| + | |||
| + | Saccharase or also called invertase is also active in the mash. This enzyme breaks sucrose into fructose and glucose but because the sucrose content in malt is insignificant the activity of this enzyme has little impact on the resulting wort composition. | ||
The consumption of one water molecule means that the production of 1 kg maltose (or an equivalent mix of glucose, maltose and larger sugars/dextrines) from starch consumes about 50 ml of water. A typical mash for a 19 liter (5 gal) batch that contains about 3 kg of starch which is converted to mostly fermentable sugars will consume about 150 ml water. This is in the order of 1% of the water added to the mash and generally neglected when calculating the amount of water needed for brewing. | The consumption of one water molecule means that the production of 1 kg maltose (or an equivalent mix of glucose, maltose and larger sugars/dextrines) from starch consumes about 50 ml of water. A typical mash for a 19 liter (5 gal) batch that contains about 3 kg of starch which is converted to mostly fermentable sugars will consume about 150 ml water. This is in the order of 1% of the water added to the mash and generally neglected when calculating the amount of water needed for brewing. | ||
| Line 43: | Line 72: | ||
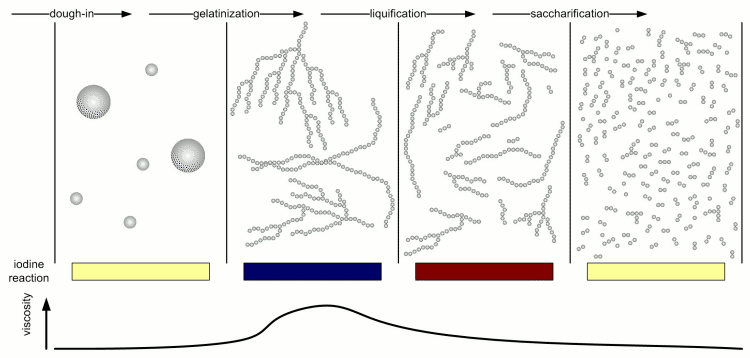
[[Image:Stages_of_starch_conversion.gif|frame|center|Figure 4 – The 4 stages of starch conversion in the mash and the progression of the iodine reaction and mash liquid viscosity.]] | [[Image:Stages_of_starch_conversion.gif|frame|center|Figure 4 – The 4 stages of starch conversion in the mash and the progression of the iodine reaction and mash liquid viscosity.]] | ||
| − | Kunze describes 4 different stages of starch conversion in the mash [Kunze, 2007] (Figure 4). Assuming a low enough dough-in temperature after dough in the starch is present as a suspension of starch granules in water. Once agitation is stopped these starch granules quickly settle. No iodine reaction can be detected yet and the viscosity of the liquid is low. But Amylase enzymes may already attack the starch granules and free and convert some starch. As the temperature is increased the gelatinization temperature of the starch is reached and the granules release their amylose and amylopectin. These large molecules quickly thicken the mash and its viscosity rises sharply. The iodine reaction will be blue-black at this stage. But at this point the amylase (especially the α-amylase) and limit dextrinase enzymes will quickly break the large amylose and amylopectin molecules into large dextrins which greatly reduces the viscosity of the mash. The brewer calls this liquification of the mash. The iodine reaction becomes less blue and more reddish brown as an indication that the size of the glucose chains is reduced. As the dextrines become smaller β-amylase and limit dextrinase become more important in efficiently reducing them to mostly fermentable sugars as the α-amylase only shows a low affinity towards these smaller dextrines. This stage is called saccharification and is completed when the iodine | + | Kunze describes 4 different stages of starch conversion in the mash [Kunze, 2007] (Figure 4). Assuming a low enough dough-in temperature after dough in the starch is present as a suspension of starch granules in water. Once agitation is stopped these starch granules quickly settle. No iodine reaction can be detected yet and the viscosity of the liquid is low. But Amylase enzymes may already attack the starch granules and free and convert some starch. As the temperature is increased the gelatinization temperature of the starch is reached and the granules release their amylose and amylopectin. These large molecules quickly thicken the mash and its viscosity rises sharply. The iodine reaction will be blue-black at this stage. But at this point the amylase (especially the α-amylase) and limit dextrinase enzymes will quickly break the large amylose and amylopectin molecules into large dextrins which greatly reduces the viscosity of the mash. The brewer calls this liquification of the mash. The iodine reaction becomes less blue and more reddish brown as an indication that the size of the glucose chains is reduced. As the dextrines become smaller β-amylase and limit dextrinase become more important in efficiently reducing them to mostly fermentable sugars as the α-amylase only shows a low affinity towards these smaller dextrines. This stage is called saccharification and is completed when the reaction with iodine disappears completely. |
<div style="clear:both;"></div> | <div style="clear:both;"></div> | ||
| Line 56: | Line 85: | ||
{| align="right" border="1" | {| align="right" border="1" | ||
| − | |+Table | + | |+Table 2 - Temperature and pH optima for starch converting mash enzymes [Narziss, 2005], [Kessler, 2006] |
|- | |- | ||
! rowspan="2" | Enzymne !! colspan="2" | optimum temperature !! optimum pH | ! rowspan="2" | Enzymne !! colspan="2" | optimum temperature !! optimum pH | ||
| Line 84: | Line 113: | ||
While it has been shown that mashes at 55C (131F) can recover up to 90% of the potential extract even though they are well below the gelatinization temperature of the starch in the grist the temperature(s) of the saccharification rest(s) are generally chosen such that the starch is able to gelatenize. This allows for a more effective use of the enzymes. | While it has been shown that mashes at 55C (131F) can recover up to 90% of the potential extract even though they are well below the gelatinization temperature of the starch in the grist the temperature(s) of the saccharification rest(s) are generally chosen such that the starch is able to gelatenize. This allows for a more effective use of the enzymes. | ||
| − | The mash temperature and time has a direct impact on the fermentability of the wort produced by mashing. This fermentability (or attenuation potential) is the ratio between the fermentable sugars (mainly glucose, maltose and maltotriose) to the total amount of extract. Since most of the extract are carbohydrates anyway the fermentability is directly linked to the balance between fermentable and unfermentable starch degradation products. And this balance is controlled by controlling the intensity of the β-amylase and limit dextrinase activity. Both these enzymes are more heat liable than α-amylase (see Table | + | The mash temperature and time has a direct impact on the fermentability of the wort produced by mashing. This fermentability (or attenuation potential) is the ratio between the fermentable sugars (mainly glucose, maltose and maltotriose) to the total amount of extract. Since most of the extract are carbohydrates anyway the fermentability is directly linked to the balance between fermentable and unfermentable starch degradation products. And this balance is controlled by controlling the intensity of the β-amylase and limit dextrinase activity. Both these enzymes are more heat liable than α-amylase (see Table 2) and this fact is used in controlling their activity especially in isothermal mashes. |
| + | The longer the β-amylase and limit dextrinase are allowed to work the more fermentable the resulting wort will be. In isothermal mashes this is controlled by the temperature. The higher the temperature the faster the β-amylase will be denatured and the less fermentable sugars are produced. At lower temperatures these enzymes will be able to work for a longer time and will produce more fermentable sugars. But because of the lower temperature these reactions will take longer and a longer mash may be necessary to achieve a sufficient conversion of the starch. Note that some α-amylase activity is necessary to provide enough non-reducing ends (especially beyond the branch points in amylopectin) for the β-amylase but that even at temperatures below the α-amylase's optimum enough activity already exists. A dependency between wort fermentability and rest temperature has been shown in the [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#Temperature|Mashing Experiments]]. In multi step saccharification rests like the German Hochkurz mash, the fermentability is mostly controlled through the length of the maltose rest which is held at 60-64 C (140-148F). | ||
{| align="right" border="1" | {| align="right" border="1" | ||
| − | |+Table | + | |+Table 3 - Temperature optima for some wort quality parameters from mashes carried out for 2-3 hrs [Briggs, 2004] (various sources) |
|- | |- | ||
! quality parameter !! Celsius !! Fahrenheit | ! quality parameter !! Celsius !! Fahrenheit | ||
| Line 106: | Line 136: | ||
|} | |} | ||
| − | |||
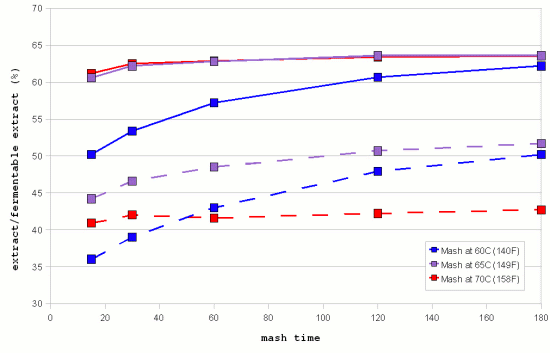
| − | [[Image:Windisch_data_mash_time_and_extract.gif|frame|right|Figure 6 - extract and fermentable extract achieved with isothermal mashes at 3 different temperatures. (Data by Windisch, Kolbach and Schild via [Briggs, 2004]]] | + | [[Image:Windisch_data_mash_time_and_extract.gif|frame|right|Figure 6 - extract (solid lines) and fermentable extract (dashed lines) achieved with isothermal mashes at 3 different temperatures. (Data by Windisch, Kolbach and Schild via [Briggs, 2004]]] |
Figure 6 shows the progress of extract and fermentable extract in isothermal mashes at 3 different temperatures. It is evident that the higher temperature mashes quickly reach a saturation of the produced extract. This is a result of the fact that the starch available for conversion is limited and the fact that there are more than enough enzymes (largely α-amylase) to convert it into soluble compounds. Only for the 60C mash does the rise in extract lag significantly behind. This is the result of only partial starch gelatinization and slower enzymatic reaction speed at this temperature. But it keeps rising and should eventually approach the levels that were reached faster by the higher temperature mashes. | Figure 6 shows the progress of extract and fermentable extract in isothermal mashes at 3 different temperatures. It is evident that the higher temperature mashes quickly reach a saturation of the produced extract. This is a result of the fact that the starch available for conversion is limited and the fact that there are more than enough enzymes (largely α-amylase) to convert it into soluble compounds. Only for the 60C mash does the rise in extract lag significantly behind. This is the result of only partial starch gelatinization and slower enzymatic reaction speed at this temperature. But it keeps rising and should eventually approach the levels that were reached faster by the higher temperature mashes. | ||
| Line 144: | Line 173: | ||
As discussed in [[Enzymes#The_effect_of_pH|Enzymes - The effect of pH]] the pH of the mash can have a significant impact on the enzyme activity and since mashing is mostly an enzymatic reactions it requires the pH to be in an acceptable range. Within that range the pH can also be used to control the reaction of the individual enzymes. | As discussed in [[Enzymes#The_effect_of_pH|Enzymes - The effect of pH]] the pH of the mash can have a significant impact on the enzyme activity and since mashing is mostly an enzymatic reactions it requires the pH to be in an acceptable range. Within that range the pH can also be used to control the reaction of the individual enzymes. | ||
| − | [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#mash_results|Mashing experiments]] Have shown that an | + | [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#mash_results|Mashing experiments]] Have shown that an suboptimal pH results in lower extract production and less fermentable wort. The optimal mash pH ranges are shown in Table 3. |
| − | A major confusion that commonly arises in the discussion of mash pH is that it changes with temperature. Mashes behave like weak acids and they disassociate more (i.e. free more H<sup>+</sup> ions) as the temperature rises. It has been shown that the pH of a mash at 65C is about 0.35 pH units lower and about 0.45 units lower at mash out temperatures (75C) compared to its pH at room temperature (25C) [Briggs, 2004]. The pH optimum of α-amylase has been determined at 5.3 in room temperature experiments. But it mashing its optimum is commonly reported to be 5.7. The reason for this is that the mash pH is commonly measured in a cooled sample of the mash. Measurement at mash temperatures is possible but common pH testing equipment like test strips and pH meters are designed for pH measurement in cooled samples. | + | A major source of confusion that commonly arises in the discussion of mash pH is that it changes with temperature. Mashes behave like weak acids and they disassociate more (i.e. free more H<sup>+</sup> ions) as the temperature rises. It has been shown that the pH of a mash at 65C is about 0.35 pH units lower and about 0.45 units lower at mash out temperatures (75C) compared to its pH at room temperature (25C) [Briggs, 2004]. The pH optimum of α-amylase has been determined at 5.3 in room temperature experiments. But it mashing its optimum is commonly reported to be 5.7. The reason for this is that the mash pH is commonly measured in a cooled sample of the mash. Measurement at mash temperatures is possible but common pH testing equipment like test strips and pH meters are designed for pH measurement in cooled samples. |
The proper mash pH does more than allowing for optimal enzyme activity it also provides the basis for the boil and cast-out wort pH. The boil pH tends to be at or slightly higher than the mash pH and the cast-out wort pH is 0.1 - 0.2 units lower than the boil pH. | The proper mash pH does more than allowing for optimal enzyme activity it also provides the basis for the boil and cast-out wort pH. The boil pH tends to be at or slightly higher than the mash pH and the cast-out wort pH is 0.1 - 0.2 units lower than the boil pH. | ||
| Line 160: | Line 189: | ||
The concentration of the mash (water to grist ratio) can have a significant impact on the mash performance. Very thick mashes ( < 2 l/kg or 1 qt/lb) are difficult to stir and extract recoveries are reduced while starch conversion is slowed [Briggs, 2004]. | The concentration of the mash (water to grist ratio) can have a significant impact on the mash performance. Very thick mashes ( < 2 l/kg or 1 qt/lb) are difficult to stir and extract recoveries are reduced while starch conversion is slowed [Briggs, 2004]. | ||
| − | A wide range of mash concentrations may be used in brewing. Traditional English mashes for example tend to be rather thick 2-2.5 l/kg | + | A wide range of mash concentrations may be used in brewing. Traditional English mashes for example tend to be rather thick (2-2.5 l/kg; 1 - 1.25 qt/lb) while German mashes tend to be on the thinner side (3.5 - 5 l/kg; 1.75 - 2.5 qt/lb). One reason for the difference is the equipment that these mashes are used in. Traditional English brewing uses a single unheated mash tun that was also used for lautering while German brewers used directly heated mash vessels that require stirring the mash. The mash also has to be pumped from and to a decoction vessel and the lauter tun. |
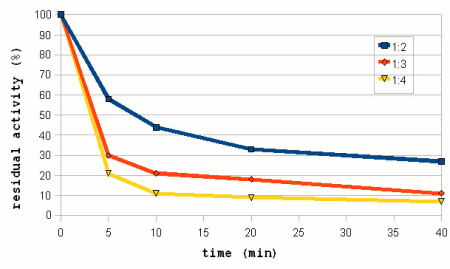
| − | The amylase enzymes are more stable in thicker mashes. Which is especially important to the more heat liable β-amylase and as a result thicker mashes give more fermentable worts than thinner mashes when mashing at high mashing temperatures [Briggs, 2004]. But while thick mashes offer better protection for the enzymes, they also inhibit the enzymatic activity through the reduced availability of free water and the sugars acting as competitive inhibitors [Briggs, 2004]. In addition to that the gelatinization of starch is also slower and happens at higher temperatures in thick mashes and as a result thinner mashes are known to give more fermentable worts at normal mashing temperatures. | + | The amylase enzymes are more stable in thicker mashes (Figure 8). Which is especially important to the more heat liable β-amylase and as a result thicker mashes give more fermentable worts than thinner mashes when mashing at high mashing temperatures [Briggs, 2004]. But while thick mashes offer better protection for the enzymes, they also inhibit the enzymatic activity through the reduced availability of free water and the sugars acting as competitive inhibitors [Briggs, 2004]. In addition to that the gelatinization of starch is also slower and happens at higher temperatures in thick mashes and as a result thinner mashes are known to give more fermentable worts at normal mashing temperatures. |
| − | But in [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#Mash_thickness|mash experiments]] little to know dependency of the fermentability on the mash thickness | + | Figure 7 shows data from mash experiments done by Windisch, Kolbach and Schild. It shows how the thinner mashes were able to convert more of the malts starch and also produce more fermentable sugars. But the ratio between fermentable extract and total extract (i.e. fermentability) remained largely constant over the range of mash thicknesses that were tested. Also note the data for the permanently soluble nitrogen. This data uses the right hand scale of the chart and shows that ticker mashes show better protoelytic activity. But that will be covered in the section about [[Protein breakdown]]. |
| + | |||
| + | In addition to that the | ||
| + | [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#Mash_thickness|mash experiments]] also showed little to know dependency of the fermentability on the mash thickness. Which matches Narziss' findings who states that the mash thickness has little impact on fermentability when well modified malts are used [Narziss, 2005]. But it was found that the mash thickness showed a strong effect on the conversion efficiency in the mash with the thinner mash being able to convert more of the starches than the thicker mash. This supports the theory that starch conversion is easier and quicker in thinner mashes. | ||
==preparation of the grist== | ==preparation of the grist== | ||
| Line 174: | Line 206: | ||
=References= | =References= | ||
| − | :[ | + | :[Briggs, 2004] Dennis E. Briggs, Chris A. Boulton, Peter A. Brookes, Roger Stevens, ''Brewing Science and Practice'', Published by Woodhead Publishing, 2004 |
| − | + | ||
:[Kunze, 2007] Wolfgang Kunze, ''Technologie Brauer und Maelzer'', 9. Auflage, VLB Berlin | :[Kunze, 2007] Wolfgang Kunze, ''Technologie Brauer und Maelzer'', 9. Auflage, VLB Berlin | ||
:[Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, ''Abriss der Bierbrauerei'', Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan). WILEY-VCH Verlags GmbH Weinheim Germany, 2005 | :[Narziss, 2005] Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, ''Abriss der Bierbrauerei'', Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan). WILEY-VCH Verlags GmbH Weinheim Germany, 2005 | ||
| Line 182: | Line 213: | ||
:[Kessler, 2008] Dr.-Ing. Matthias Thaddäus Keßler, ''Analytische Erfassung und Interpretation der Stärkedegradation im Gersten- und Malzkorn und die Aussagekraft für den Brauprozess'', Dissertation, Technische Universität München, 2006 | :[Kessler, 2008] Dr.-Ing. Matthias Thaddäus Keßler, ''Analytische Erfassung und Interpretation der Stärkedegradation im Gersten- und Malzkorn und die Aussagekraft für den Brauprozess'', Dissertation, Technische Universität München, 2006 | ||
:[TU Vienna] [http://www.vt.tuwien.ac.at/scripts/172942/172942_Brauerei_04.pdf www.vt.tuwien.ac.at/scripts/172942/172942_Brauerei_04.pdf] | :[TU Vienna] [http://www.vt.tuwien.ac.at/scripts/172942/172942_Brauerei_04.pdf www.vt.tuwien.ac.at/scripts/172942/172942_Brauerei_04.pdf] | ||
| + | :[Valclavik] Vickie A. Valclavik, Elizabeth W. Christian, ''Essentials of Food Science'', Third Edition, Springer | ||
| + | :[Champe] Pamela C, Champe, Richard A. Harvey, Denise R. Ferrier, ''Biochemistry'', Lippincott's Illustrated Reviews | ||
| + | |||
| + | |} | ||
Latest revision as of 03:45, 1 December 2010
|
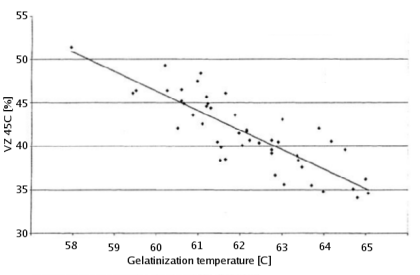
90-92% of the solids in brewing wort are carbohydrates [Briggs, 2004]. Proteins make up only 4-5% and the rest are vitamins, minerals and trace elements. Of the carbohydrates more than 95% are products of the starch conversion that happens in the mash tun. As a result the starch degradation is the main purpose of mashing. It produces the fuel that the yeast needs for fermentation from the insoluble starch found in malt and mash tun adjuncts. The extend to which this conversion happens will determine the efficiency with which the ingredients are utilized and the fermentability of the produced wort as well as other quality characteristics. In mashing starch conversion is preceded by gelatinization of the starches. While this is not necessary for conversion, as plants do exactly this when they germinate, it greatly speeds up the process through the exposure of a lot of substrate (amylose and amylopectin) from the starch granule to the starch converting enzymes (mainly α-, β-amylase and limit dextrinase). ContentsGelatinizationStarch granules are insoluble in cold water and will absorb only little water. They form a suspension that quickly settles once agitation stops. As the water is heated (50+ C) more and more water is absorbed and the granules start to swell [Narziss, 2005]. The water absorbed during this process can be up to 30 times the weight of the starch granule. This uptake of water initially happens within the amorphous growth rings. At this point the granule starts to leach amylose and the crystalline layers break open and separate from the starch granule as gelatinous sheets. At this point the crystalline structure is lost and the process becomes irreversible with respect to the shape of the starch granule [Shetty, 2006]. The starch granule has gelatinized. While the temperature range during with gelatinization occurs has been found to be quite narrow for individual starch granules (~ 1C) the temperature range between the gelatinization of the first granules and the complete gelatinization of all granules can be quite large. Figure 1 shows the temperature ranges for gelatinization for a number of starches. It can be seen that not all these starches fully gelatinize at temperatures that are encountered in a saccharification rest. If this is not the case they will have to be gelatinized before that rest though either a cereal mash or the use of pre-gelatinized (e.g. flaked) forms. Another interesting aspect is the different gelatinization temperature ranges that have been determined for large and small barley granules. 90% of the starch in barley are large granules which will be gelatinized at saccharification rest temperatures while the rest are small starch granules which may not fully gelatinize until higher temperatures are reached. This can explain some of the efficiency benefits that can be gained from a mash-out or a decoction mash. Gelatinization is a process that requires free water for the swelling and breaking the hydrogen bonds that hold the crystalline structures in place. If free water is limited due to a high concentration of starch (e.g. overly thick mash conditions) less swelling takes place and a melting of the crystalline section needs to occur [Donald, 2004]. This leads to an increase in the gelatinization temperature. A limitation of free water can also be caused by the presence of sugars other dissolved solids. For corn starch, for example, it has been shown that a 25% sucrose solution increases the gelatinization temperature from 70 to 78C [Donald, 2004]. This is assumed to be one of the factors why thick mashes showed a lower efficiency compared to thin mashes in the Mashing Experiments. As starch starts to gelatinize the viscosity of the liquid will increase. This can be noticed in brewing to some extend but by far less that what is commonly seen in cooking. The reason for that is the presence of enzymes (in particular α-amylase) that will start breaking down the amylose and amylopectin molecules as soon as they become accessible. This process reduces the viscosity of the mash and is therefore called liquification [Kunze, 2007]. This effect is used for mitigating the risk of scorching the mash during a decoction boil by resting them at 70-74C before continuing to heat them to a boil. A strong increase in viscosity can however become a problem in cereal mashes. Especially when using rice starch which is known to swell very intensely. This can lead to scorching or even the immobilization of mash agitators [Kunze, 2007]. To counteract that some malt should be added to cereal mashes and a short liquification rest might be held between 75 and 80C (just before all the α-amylase gets denatured) before it is then heated to boiling. The gelatinization temperature also depends on growing conditions and crop year [Kunze, 2007]. And Kessler showed that a reasonable correlation exists between the VZ 45C malt analysis number and the gelatinization temperature [Kessler, 2008]. VZ 45C is the ratio between the extract that can be extracted through mashing at 45C and the amount that can be extracted with a congress mash. This number is given on some (mainly German) malt analysis sheets. Figure 2 shows the data that was published by Kessler. According to Weyermann the VZ45 for their malts can be as low as 35. This may result in a gelatinization temperature as high as 65C. While this temperature should not be a problem when using a single saccharification rest, it can become problematic when a maltose rest is held at 63C at which temp the starch will not be fully gelatinized. If this is the case an extended rest at 65C needs to be held in order to achieve the desired fermentability of the produced wort. Enzymatic starch breakdownStarch conversion in the mash is mainly an enzymatic process and there are 4 types of enzymes that can take part. The 2 best known enzymes are α- and β-amylase. Both work on the α(1-4) glycosidic bonds of the starch and dextrin molecules. Another enzyme is limit dextrinase which is able to break the α(1-6) glycosidic bond that forms the branch points in the amylopectin molecule. At last there is maltase, an enzyme that can cleave a glucose molecule from the non-reducing end of a disaccharide or polydisaccharide. But because this enzyme is quickly deactivated by temperatures above 45 C, it generally doesn't play any significant role in mashing. α-amylase is an enzyme that can cleave any α(1-4) bond in starch and dextrines except for the ones close to the branch points. This breakage of a glycosidic bond is also called hydrolyzation as it consumes one water molecule. It is the enzyme that is responsible for the rapid loss of viscosity and iodine reaction after malt starch gelatinized. A process that is called liquefaction of the mash. While α-amylase is very good at breaking down starch into smaller dextrines, which quickly reduces the iodine reaction from a black-blue to a more reddisch brown color, it is not very good at producing fermentable sugars. This results from the low affinity that α-amylase has towards smaller glucose chains.
α- and β-amylase alone cannot completely saccharify (i.e. convert to all sugar) starch. The reason for that are α(1-6) that make up 6-7% of the bonds in amylopectin. But malt contains one enzyme that can: limit dextrinase. This enzyme has with 06-62.5C a similar temperature optimum as β-amylase. It's instability at common mash temps (63-70C) is the reason why most brewing wort will contain a large number of limit dextrines which are the branch points that were left behind by α- and β-amylase activity. Unless a very high fermentability is desired the residual dextrines are actually desired as they contribute positively to the character of the beer. Maltase is an enzyme that has its optimum between 30 and 40 C and can split a single glucose molecule from the non-reducing end of a glucose chain (similar to β-amylase which splits a glucose pair from the non-reducing end of glucose chains). It's affinity to the substrate increases as the degree of polymerization decreases and it is highest for maltose (degree of polymerization is 2) [Kessler, 2006]. But it is generally of little interest in mashing as at its working temperature there is not much maltose present in the wort (which assumes that the mash is doughed in at or below 40 C). If activity of this enzyme is desired to increase the glucose level of the wort the mash needs to be held for saccharification at 63-65C and after having been cooled to 40 C fresh malt is added which also adds new maltase enzymes. After a rest of 30-45 min the mash is heated again to convert the starch that has been added with the new malt. This mash schedule has been introduced by Markus Hermann from the Weihenstephan brewing school to produce high glucose worts for ester rich Weissbiers [Hermann, 2005] Saccharase or also called invertase is also active in the mash. This enzyme breaks sucrose into fructose and glucose but because the sucrose content in malt is insignificant the activity of this enzyme has little impact on the resulting wort composition. The consumption of one water molecule means that the production of 1 kg maltose (or an equivalent mix of glucose, maltose and larger sugars/dextrines) from starch consumes about 50 ml of water. A typical mash for a 19 liter (5 gal) batch that contains about 3 kg of starch which is converted to mostly fermentable sugars will consume about 150 ml water. This is in the order of 1% of the water added to the mash and generally neglected when calculating the amount of water needed for brewing. Kunze describes 4 different stages of starch conversion in the mash [Kunze, 2007] (Figure 4). Assuming a low enough dough-in temperature after dough in the starch is present as a suspension of starch granules in water. Once agitation is stopped these starch granules quickly settle. No iodine reaction can be detected yet and the viscosity of the liquid is low. But Amylase enzymes may already attack the starch granules and free and convert some starch. As the temperature is increased the gelatinization temperature of the starch is reached and the granules release their amylose and amylopectin. These large molecules quickly thicken the mash and its viscosity rises sharply. The iodine reaction will be blue-black at this stage. But at this point the amylase (especially the α-amylase) and limit dextrinase enzymes will quickly break the large amylose and amylopectin molecules into large dextrins which greatly reduces the viscosity of the mash. The brewer calls this liquification of the mash. The iodine reaction becomes less blue and more reddish brown as an indication that the size of the glucose chains is reduced. As the dextrines become smaller β-amylase and limit dextrinase become more important in efficiently reducing them to mostly fermentable sugars as the α-amylase only shows a low affinity towards these smaller dextrines. This stage is called saccharification and is completed when the reaction with iodine disappears completely. Effect of mashing conditionsMashing conditions like temperature, time, water/grist ratio pH and others affect the complex interactions between the enzymes and the substrate and have an effect on the quality of the produced wort. Through a control of these conditions the brewer can greatly influence the wort composition and the final character of the beer. In addition to that mashing conditions also have an effect on the amount of extract that is created from the grist and will determine how well it is utilized. These are reasons why a brewer should have a good understanding on how mashing effects the wort and later the beer. The following sections will evaluate the effect of the various mash parameters in detail. Temperature and Time
While it has been shown that mashes at 55C (131F) can recover up to 90% of the potential extract even though they are well below the gelatinization temperature of the starch in the grist the temperature(s) of the saccharification rest(s) are generally chosen such that the starch is able to gelatenize. This allows for a more effective use of the enzymes. The mash temperature and time has a direct impact on the fermentability of the wort produced by mashing. This fermentability (or attenuation potential) is the ratio between the fermentable sugars (mainly glucose, maltose and maltotriose) to the total amount of extract. Since most of the extract are carbohydrates anyway the fermentability is directly linked to the balance between fermentable and unfermentable starch degradation products. And this balance is controlled by controlling the intensity of the β-amylase and limit dextrinase activity. Both these enzymes are more heat liable than α-amylase (see Table 2) and this fact is used in controlling their activity especially in isothermal mashes. The longer the β-amylase and limit dextrinase are allowed to work the more fermentable the resulting wort will be. In isothermal mashes this is controlled by the temperature. The higher the temperature the faster the β-amylase will be denatured and the less fermentable sugars are produced. At lower temperatures these enzymes will be able to work for a longer time and will produce more fermentable sugars. But because of the lower temperature these reactions will take longer and a longer mash may be necessary to achieve a sufficient conversion of the starch. Note that some α-amylase activity is necessary to provide enough non-reducing ends (especially beyond the branch points in amylopectin) for the β-amylase but that even at temperatures below the α-amylase's optimum enough activity already exists. A dependency between wort fermentability and rest temperature has been shown in the Mashing Experiments. In multi step saccharification rests like the German Hochkurz mash, the fermentability is mostly controlled through the length of the maltose rest which is held at 60-64 C (140-148F).
Figure 6 shows the progress of extract and fermentable extract in isothermal mashes at 3 different temperatures. It is evident that the higher temperature mashes quickly reach a saturation of the produced extract. This is a result of the fact that the starch available for conversion is limited and the fact that there are more than enough enzymes (largely α-amylase) to convert it into soluble compounds. Only for the 60C mash does the rise in extract lag significantly behind. This is the result of only partial starch gelatinization and slower enzymatic reaction speed at this temperature. But it keeps rising and should eventually approach the levels that were reached faster by the higher temperature mashes. The fermentable extract shows a different dependency on the temperature. The higher the mash temperature the quicker the level of fermentable extract levels off and remains constant. This is a result of the quicker denaturing of the β-amylase and limit dextrinase. After 180 min mashing the mash at 65C still leads the mash at 60C with respect to the amount of fermentable extract generated but it starts to level off and the mash at 60C is expected to generate more fermentable extract than the mash at 65C if the mashing time is extended past 180F. While the discussion was for idealized enzymatic reactions, which are different from what is happening in the mash the Temperature section of the Enzymes article shows graphs that are very similar to the data shown in Figure 4 which supports the theory that the fermentability is controlled through the time that the β-amylase and limit dextrinase are allowed to act. pH and brewing water
Mashing experiments Have shown that an suboptimal pH results in lower extract production and less fermentable wort. The optimal mash pH ranges are shown in Table 3. A major source of confusion that commonly arises in the discussion of mash pH is that it changes with temperature. Mashes behave like weak acids and they disassociate more (i.e. free more H+ ions) as the temperature rises. It has been shown that the pH of a mash at 65C is about 0.35 pH units lower and about 0.45 units lower at mash out temperatures (75C) compared to its pH at room temperature (25C) [Briggs, 2004]. The pH optimum of α-amylase has been determined at 5.3 in room temperature experiments. But it mashing its optimum is commonly reported to be 5.7. The reason for this is that the mash pH is commonly measured in a cooled sample of the mash. Measurement at mash temperatures is possible but common pH testing equipment like test strips and pH meters are designed for pH measurement in cooled samples. The proper mash pH does more than allowing for optimal enzyme activity it also provides the basis for the boil and cast-out wort pH. The boil pH tends to be at or slightly higher than the mash pH and the cast-out wort pH is 0.1 - 0.2 units lower than the boil pH. α-amylase is stabilized through the presence of calcium ions in the mash [Briggs, 2004]. While this should have an effect on the amount of starch that is converted in mashing it was not observed in mashing experiments. Most likely because α-amylase is still plenty stable at common mashing temperatures. This effect can however be used in cereal mashing where it is desired to keep the α-amylase active until all of the starch is gelatinized in order to limit the increase in viscosity as much as possible. β-amylase stability however is not effected by Calcium ions and the concentration of calcium in the mash has therefore no effect on the amount of fermentability of the produced wort. water to grist ratio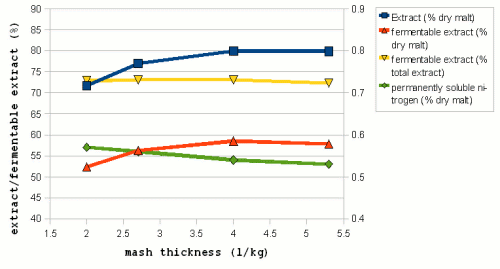 Figure 7 – The effect of mash thickness on extract (■), fermetable extract (▲), fermetability (▼) and permanently soluble nitrogen (♦) (fraction of proteins small enough to survive into the pitched wort)for mashes made a 60C (140F) for a duration of 180 min. The permanently soluble nitrogen fraction uses the scale on the right hand side (0.4 – 0.9%) (Data by Windisch, Kolbach and Schild via [Briggs, 2004]) The concentration of the mash (water to grist ratio) can have a significant impact on the mash performance. Very thick mashes ( < 2 l/kg or 1 qt/lb) are difficult to stir and extract recoveries are reduced while starch conversion is slowed [Briggs, 2004]. A wide range of mash concentrations may be used in brewing. Traditional English mashes for example tend to be rather thick (2-2.5 l/kg; 1 - 1.25 qt/lb) while German mashes tend to be on the thinner side (3.5 - 5 l/kg; 1.75 - 2.5 qt/lb). One reason for the difference is the equipment that these mashes are used in. Traditional English brewing uses a single unheated mash tun that was also used for lautering while German brewers used directly heated mash vessels that require stirring the mash. The mash also has to be pumped from and to a decoction vessel and the lauter tun. The amylase enzymes are more stable in thicker mashes (Figure 8). Which is especially important to the more heat liable β-amylase and as a result thicker mashes give more fermentable worts than thinner mashes when mashing at high mashing temperatures [Briggs, 2004]. But while thick mashes offer better protection for the enzymes, they also inhibit the enzymatic activity through the reduced availability of free water and the sugars acting as competitive inhibitors [Briggs, 2004]. In addition to that the gelatinization of starch is also slower and happens at higher temperatures in thick mashes and as a result thinner mashes are known to give more fermentable worts at normal mashing temperatures. Figure 7 shows data from mash experiments done by Windisch, Kolbach and Schild. It shows how the thinner mashes were able to convert more of the malts starch and also produce more fermentable sugars. But the ratio between fermentable extract and total extract (i.e. fermentability) remained largely constant over the range of mash thicknesses that were tested. Also note the data for the permanently soluble nitrogen. This data uses the right hand scale of the chart and shows that ticker mashes show better protoelytic activity. But that will be covered in the section about Protein breakdown. In addition to that the mash experiments also showed little to know dependency of the fermentability on the mash thickness. Which matches Narziss' findings who states that the mash thickness has little impact on fermentability when well modified malts are used [Narziss, 2005]. But it was found that the mash thickness showed a strong effect on the conversion efficiency in the mash with the thinner mash being able to convert more of the starches than the thicker mash. This supports the theory that starch conversion is easier and quicker in thinner mashes. preparation of the gristThe way the grist is prepared for mashing can have a substantial impact on the mash performance. Of particular importance is the level of crushing that the malt was subjected to. A finer crush results in more flour and smaller grist particles. The endosperm is also more completely separated from the husks. All this gives the brewing water and the enzymes faster and better access to the starch which results in faster conversion, greater extract recoveries (i.e. better conversion efficiency) and higher wort fermentability. Because of that brewers generally prefer the malt to be crushed as fine as their system (in particular the lauter system) allows. Some commercial breweries use pulverized grists but they have to use mash filters instead of lauter tuns since the pulverization of the grist also destroyed the husks which are necessary as a filter support in lauter tuns. While an increase of the extract recovery through a finer crush was shown in mashing experiments an effect on the fermentability was not evident. References
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||