Difference between revisions of "How pH affects brewing"
m (→hop utilization) |
m (→Hop utilization) |
||
| Line 122: | Line 122: | ||
=Hop utilization= | =Hop utilization= | ||
| − | [Image:Alpha_acid_solubility.gif|frame|right] | + | [[Image:Alpha_acid_solubility.gif|frame|right]] |
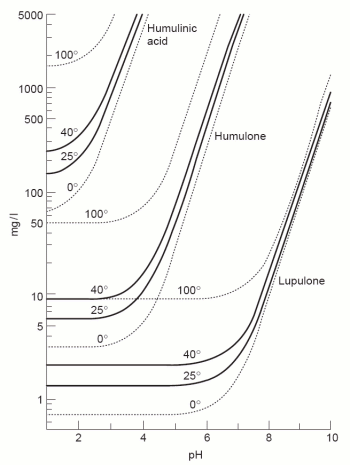
Before the hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved in boiling wort. The solubility depends on both temperature and pH of that wort. Except for elevation and equipment dependent variations the boil temperature is largely constant. But the pH of the boil can easily vary and with it the solubility of the hop acids. It has been shown that this solubility increases with pH [Briggs, 2004] which is why the bitterness extraction from the hops is greater at a higher boil pH. But many brewers have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher. | Before the hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved in boiling wort. The solubility depends on both temperature and pH of that wort. Except for elevation and equipment dependent variations the boil temperature is largely constant. But the pH of the boil can easily vary and with it the solubility of the hop acids. It has been shown that this solubility increases with pH [Briggs, 2004] which is why the bitterness extraction from the hops is greater at a higher boil pH. But many brewers have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher. | ||
Revision as of 01:11, 22 September 2009
|
This is the 2nd part in a 3 part series that takes the interested brewer through the pH in brewing. The 1st part (An Overview of pH) explained the basics that are needed to understand this and the following part. This part details how pH affects various stages in the brewing process. The 3rd part explains in detail how the mash pH can be affected such that it is at the targeted level.
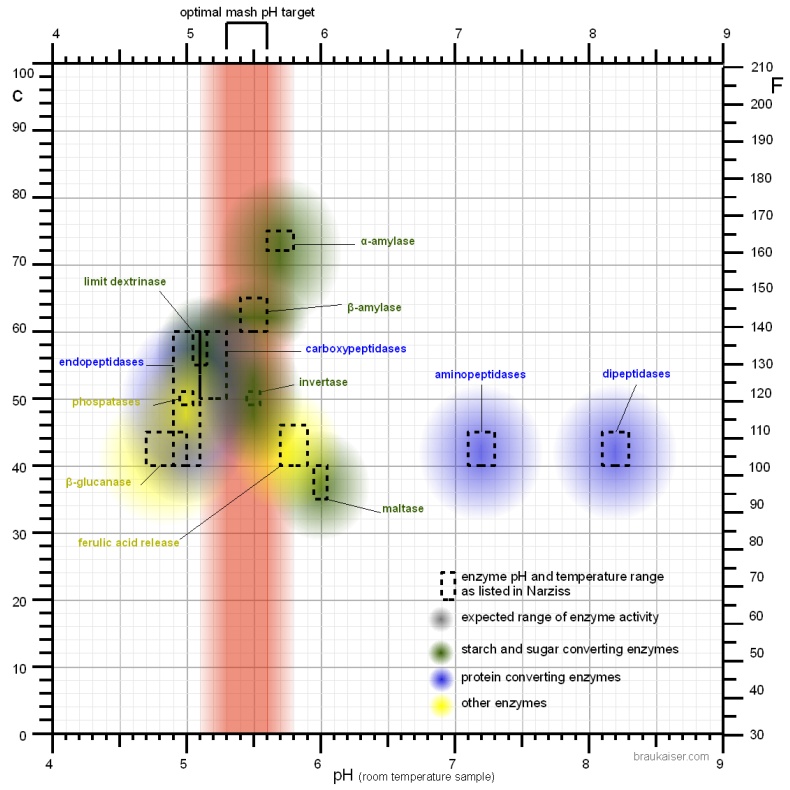
In brewing pH affects: Contents[hide]enzymatic activity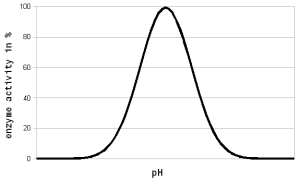 Figure 1 - For each enzyme there is an optimal pH at which it works best. The location and width of that optimum depends on the enzyme. The pH dependent activity change is caused by changes of the enzymatic structure and electric charges especially at or around the active sites witch hold on to the substrate during the reaction All enzymes are proteins which rely on their shape for their function. This shape is determined by the sequence of amino acids that make up the protein and bonds between these amino acids. It is also determined by the electrostatic forces between electrically changed amino acids in this chain. As we saw in the first part, the distribution of electrical charges depends on the pH of the environment. A high pH (low H+ concentration) will cause more of the amino acids (the ones that behave like weak acids) to donate more H+ into the solution which leaves the remaining amino acid negatively changed whereas a low pH (high H+ concentration) has the opposite effect. Some amino acids (the ones that behave like weak bases) will accept H+ from the solution and therefore change to having a positive charge as the pH falls. In order for enzymes to function a proper balance of these charged amino acids needs to be achieved which will be present at and around the pH at which the enzyme is most effective. As long as the difference between the optimal and actual pH is low (1-2 pH units) the effect of pH on the enzyme activity is reversible since the enzyme is not permanently damaged (denatured). In practice this means that the pH can be corrected even after dough-in and little or no enzymes will be lost as a result of a suboptimal initial pH. But if the pH is adjusted to an adequate level these enzymes will get a later start and some of them may have already been denatured as a result of the heat before they got a chance to work at an optimal pH. This is especially true for the more heat liable beta-amylase and limit dextrinase. If the pH is substantially far from the optimum the enzyme can also be denatured. The pH sensitivity of enzymes is discussed in further detail in The Effect of pH on Enzymes In mashing we tend to target the mash pH to optimize the effectiveness of the amylase enzymes. For alpha amylase the pH optima has been found to be at 5.3 and for the beta amylase it is between 5.1 and 5.3 [Briggs, 2004] (see pH and brewing water in Starch Conversion). But when their activity is evaluated at mashing temperatures and the pH of a cooled mash sample is measured it shows their pH optima at 5.7 and 5.4-5.6 respectively. This is the result of a pH shift in the mash that happens when the mash is heated and possibly a shift of the pH optima of the enzyme. At common starch conversion temperatures (65C/150F) the pH of the mash appears 0.35 units lower than what it is for a room temperature sample of the mash [Briggs, 2004]. This needs to be taken into account when looking at pH optima for various mash enzymes. But most commonly the pH optima that are reported in the literature were determined by testing cooled mash samples. When I used a temperature correcting pH meter to test a mash at mash temp and at room temp I found that the pH shift was only ~ -0.2 pH units. This has been confirmed by other home brewers and is less than the 0.35 that have been reported by Briggs and other authors. I assume that the apparent 0.35 pH shift is the result of an actual pH shift in the mash and a shift of the pH optimum of the enzyme. Most homebrewers measure the pH of a cooled mash sample when checking pH. While pH meters are able to measure the hot mash pH it is not advisable to do so because it shortens the life of the probe. If colorpHast strips are used in the hot mash their color reaction is the same or very close to their color reaction in a room temp mash sample. This means that these strips produce the same pH reading regardless of temperature (at least when used in mash or wort). But it should be kept in mind that these strips report a pH that is about 0.3 pH lower than the actual pH colorpHast strips in brewing. Because of that it is more practical to focus on the mash pH optima that were determined with room temperature samples. A commonly accepted optimal range for the mash pH is 5.2 - 5.7 with 5.5 being optimal for starch conversion activity but many authors report wort and beer quality benefits if the pH is lowered into the 5.2 - 5.4 range [Kunze, 2007][Narziss, 2005]. Kunze in particular lists these benefits of a mash pH as low as 5.2. Some of these benefits will be explained in the following sections [Kunze, 2007]:
The pH should not fall below 5.2 since at this point the activity of the amylase enzymes starts to suffer significantly mash pH experiments. This is particularly important if sour mashing is used to create sour beers and not just as a means for adjusting mash pH. Just enough sour mash should be added to correct the pH and the rest is added towards the end of the mash when the conversion of the main mash is complete. Even a pH between 5.2-5.4 is already suboptimal for mashes with large amounts of enzymatic weak malts like Munich malts those mashes are better done with a mash pH above 5.4 which will be closer to the pH optimum of the alpha amylase enzyme. When decoction mashing is used the mash pH should not be lower than 5.4 [Kunze, 2007]. While Kunze doesn't give a reason I assume that this is because the boiling of the decoctions lowers the mash pH which can lead to too low of a mash pH for the already enzymatically weakened decoction mash. pH optima of enzymes other than the amylasesWhile the targeted mash pH is generally not determined by enzymes other than the amylase enzymes it also has an affect on other enzymes which may be considered in certain mashing schedules. If a ferulic acid rest is held at 35-40C (95-105F) the release of ferulic acid is reduced if the pH is below 5.7 [Narziss, 2005]. Because of that it is advisable to add any acidulated malt, which may be part of the grist in order to correct the pH, after that rest is completed. Very lightly kilned malts contain the enzyme phytase which is able to release phospate from phytin present in the malt [deLange, 2004]. That release of phospate serves to lower the mash pH during the so called acid rest. But not only lowers it the mash pH, it also increases the buffer capacity of the mash. This increased buffer capacity may make it later more difficult for the yeast to lower the mash pH. The enzyme has a pH optimum of about 5.0 which is the reason for the increased buffer capacity of worts produced from lightly kilned malts (e.g. Pilsner malt) with lower pH mashes. The β-glucanase, which is most active between 40 and 50 C (104 – 122 F) and degrades beta glucanes, likes a little more acidic. It’s pH optimum is between 4.7 and 5.0 [Narziss, 2005]. In malt 4 groups of protein degrading enzymes (proteases) exist. Endopeptidases are the protein equivalent of α-amylase; they break peptide bonds of proteins and peptides within the molecule. Carboxypeptdiases are like β-amylase because they break off amino acids from the carbonyl end of the protein molecules. Dipeptidases are like the enzyme maltase which breaks maltose in two glucose molecules only that they break a dipeptide into two amino acid molecules. And the last group are the aminopeptidases which act like carboxypeptidases but attack the protein from its amino end. Narziss lists the following pH optima for these groups [Narziss, 2005]:
Only endopeptidases and carboxypeptidases are active at mashing pH levels. Compared to the amylases these enzymes prefer a slightly more acidic environment which explains the higher release of amino acids in lower pH mashes. If necessary, pH can also be used to limit these protein degrading enzymes by lowering the mash pH not until the saccharification temperature range 60-70 C (140-160 F) is reached. The mash environment is too acidic for effective dipeptidase and aminopeptiodase activity. High mash pH also favors the release of colored malt compounds into the mash [Lewis&Bamforth, 2006]. This is yet another reason why it is beneficial to mash lighter beers at the lower end of the optimal mash pH range. conclusionA number of enzymes are active in the mash and many of them have different pH optima which makes it sometimes confusing to choose the proper mash pH. After presenting all that above, here are some simple guidelines that can be followed when determining which mash pH should be targeted or deciding if the current mash pH needs adjustment:
Extraction of TanninsTannins are a chemical compound that is found in vegetative matter like grain husks, hops, grape skins and tea leaves. In the brewing process the main source of tannins in the wort originates from the malt husks and the hops. If present in excess they will give the beer a dry, astringent and sometimes puckering mouthfeel. But why do some beers have a problem with tannins while others don't? This too has to do with pH. The solubility of tannins in water is affected by 2 factors. One of them is temperature and the other is pH. The effect of the temperature is obvious. The higher the temperature the more soluble most compounds including tannins are and the higher the rate at which they are leached into the wort. But the effect of pH is more profound. Polyphenols, which tannins are, are weak acids. And as we saw in acids and bases they readily donate protons to their environment if the concentration of H+ is low enough, i.e. the pH is high enough. Once a polyphenol molecule looses one or more protons it becomes positively charged. Due to the water molecule's charged nature, one side is positive and the other is negative, water is a great solvent for charged molecules and that's why the solubility of tannins increases when the pH rises [Bogspot.com, 2009]. A generally accepted pH threshold is 5.8-6.0 [source?]. As a result, the pH in the mash is generally low enough to prevent excessive tannin extraction. But sparging with highly alkaline water can quickly consume the mash's buffer capacity and lead to pH levels that create excessive tannin extraction into the wort. This is because alkaline water is a strong buffer bases on carbonates and bicarbonates. As the wort is diluted and with it the mash’s ability to buffer the pH at mash pH the sparge water and its pH are taking over which can raise the pH above 6.0 and cause excessive tannin extraction. There are a number of ways that this pH raise during the sparge can be prevented or at least mitigated such that the pH is not allowed to raise above 5.8:
Protein coagulationAs we saw with the enzyme activity and the tannin extraction the main effect that a changing pH has is its ability to change the electrical charges on molecules. And that is no different with proteins during wort boiling. But in addition to that, another structural aspect of proteins matters. Proteins are chains of amino acids that are folded in a structure that allows them to do their job in an organism. Some of these amino acids react well with water, they are called hydrophilic, while others try to avoid water, and they are called hydrophobic. In water soluble proteins the hydrophobic amino acids are oriented such that they face towards the center of the protein and therefore can react with each other rather than with the surrounding water while the hydrophilic ones face outward. When the protein coagulates it the chain of amino acids is not broken but it looses its folded structure and some of the hydrophilic amino acids will face outward. Because the hydrophilic amino acids rather react with other hydrophobic amino acids they try to avoid water by joining with the hydrophobic amino acids of other coagulated proteins. As a result the proteins clump together and form the flocks we all know as hot and cold break. In addition to that tannins from the husks and hop material, which are present in the wort, will attach themselves to these areas these hydrophobic areas, which are generally rich in the amino acid proline, via hydrogen bonds and form bridges between the coagulated proteins. But how does pH affect this? This is where the concept of the isoelectric point, which we discussed in isoelectric point, plays a role. When the overall charge on the proteins is neutral, which it is at their isoelectric point, they don't repel each other anymore. Such repulsion would be the case at a pH lower or higher than the iso-electric point where the proteins are predominantly negative or positive charged respectively. When they don't repel each other the hydrophobic sections of the denatured proteins can react with each other much more easily and they'll clump together much faster. The iso-electric point is also where the solubility of a protein is the lowest since uncharged molecules don't react with water as well as charged ones. All this helps the coagulation during the boil. The iso-electric point of wort proteins is around 4.9 [BrewingTechniques, 1993]. But a common boil pH of 5.2 – 5.4 (room temperature sample) does not reach a pH that low and therefore the protein coagulation is not as good as it could be [Narziss, 2005]. While it is possible to lower the boil pH through the addition of acids, it is generally not done because the level of protein coagulation that is achieved at a pH of 5.2 is sufficient and further reduction of the boil pH further reduces the hop alpha acid utilization from the hops. During the boil the pH drops by about 0.1 – 0.2 pH units from 5.5 – 5.3 pH to about 5.3 – 5.2 pH. This may be due to the addition of bitter acids from the hops, formation of acidic Maillard products, precipitation of alkaline phosphates or the reaction of polypeptides with calcium, liberating protons [Briggs, 2004]. Hop utilizationBefore the hop bitter acids can be transformed into their iso- forms, which are more soluble, they need to be dissolved in boiling wort. The solubility depends on both temperature and pH of that wort. Except for elevation and equipment dependent variations the boil temperature is largely constant. But the pH of the boil can easily vary and with it the solubility of the hop acids. It has been shown that this solubility increases with pH [Briggs, 2004] which is why the bitterness extraction from the hops is greater at a higher boil pH. But many brewers have reported that the quality of the bitterness extracted at high boil pH is perceived as being harsher. The reason why the solubility increased with rising pH is the same as it was for the tannin extraction. Hop resin behaves acidic and wants to loose H+ at higher pH. That leaves a charged molecule which in turn is more soluble in water. The reduction in solubility of hop acids can be seen during fermentation. After the wort is pitched with yeast and the pH falls during fermentation most of the unisomerized hop resins will come out of solution again and become part of the brown gunk the floats on top of the Kraeusen which, if a smooth bitterness is desired, should be removed via blow-off tube, skimming and not allowed to fall back into the beer [Narziss, 2005][Kunze, 2007]. Maillard reactionsMaillard reactions or non-enzymatic browning is a reaction between amino acids and reducing sugars that yields melanoidins which are responsible for the darkening of both malt during kilning and wort during boiling. The rate of Maillard reactions is affected by wort concentration, temperature and pH. Here we want to have a look at the effects of the pH during the wort boil. The higher pH the faster the rate of Maillard reactions will be. I was able to observe that during my mashing experiments when I boiled the wort from a 5.5 pH and a 6.5 pH mash. The result is shown in Figure X (insert Image:Experiment_normal_vs_high_pH.jpg). While in this case the boil time was only 15 min, a wort boil pH of 6.5 is well out of the normal range and a beer brewed with that pH will have more severe problems than just increased color. But aiming for a lower boil pH (5.3 - 5.4 at room temperature) will help in keeping lighter beers from picking up too much color during boiling. But how is the pH affecting the rate of the Maillard reactions? It is through its affect on the amino acid. As mentioned earlier, Maillard reactions start with the reaction between a reducing sugar and an amino acid. This reaction happens at a much higher rate when the amino group of the amino acid becomes more likely to donate its electrons (Nucleophilic) to the bond with the reducing sugar. Which is a result of the amino group having lost a proton (H+) or being on the edge of loosing it to counteract the low H+ concentration in the high pH environment. Higher pH -> more amino acids have lost a proton from their amino groups -> the are now more likely to donate an electron to the reducing sugar -> more Amadori products which are an intermediate to the products of Maillard reactions. nutrient uptake by the yeastShortly after being pitched into fresh wort yeast will start lowering the pH of the surrounding medium (i.e. beer). This is the result of ammonium ion and amino acid uptake, secretion of organic acids [Briggs, 2004] and most importantly a proton pump which moves H+ ions from the yeast cell into the beer. This proton pump is very important to the yeast and it is the most abundant protein in its cell membrane [Briggs, 2004]. The resulting pH gradient through the yeast's cell wall facilitates the uptake of nutrients like maltose. Maltose uptake is a proton symport process through the cell membrane. The proton concentration outside the cell is greater (lower pH) than inside the cell (higher pH) and therefore a natural gradient exists that encurages protons to flow from the outside to the inside of the cell. Though the use of a symporter, a cell membrane protein, maltose can “piggy back” on the flow of protons into the cell. This is one of the reasons why yeast cells do better in an acidic environment and have means of lowering the pH. The ability of yeast to lower the pH of the beer is important for healthy and low yeast stress fermentation and is one of the reasons why sufficient pitching rates are important and why it is better to step up starters than to start a small amount of yeast in a large starter. The more yeast cells that are working on lowering the pH the faster the pH will be able to drop. As yeasts age, starve or otherwise loose their vitality it becomes increasingly difficult for them to pump H+ from their cells into the beer. After all, this goes against natures desire to equalize everything and therefore takes energy. The result is that the pH of the beer rises slightly after primary fermentation and can rise significantly if it is not taken off the yeast before a large number of yeast cells start to autolyze. The inhibition of beer spoilage organismsBesides alcohol and hop resins, the low pH of beer, which is generally lower than 4.5, is one of the main reasons why it provides a poor growth medium for many bacteria. There are for example no pathogens (bugs that can make you sick like E. coli or C. botulinum) that can grow in beer and it is therefore safe to even drink beers that have been infected. Wort generally has a pH between 5.2 and 5.6 which allows the growth of pathogens and care should be taken if it is stored unpitched and unrefrigerated for an extended period of time. The FDA requires that all canned food stuff with a pH greater than 4.6 needs to be heat treated under pressure to allow for temperatures that are high enough for sterilization in order to eliminate pathogens and their spores. That's why wort needs to be pressure canned or frozen to be stored. clarifies in kettle and fermenterBrewers may use fining agents in the kettle and in the finished beer. Depending on the product used it aids the precipitation of proteins, yeast cells or tannins but all aim at reducing beer haze and through it improve clarity and haze stability. Here we want to focus on the clarifiers that work by attracting protein and yeast through electrostatic forces. This is the case for collagen (Gelatin or Isinglass) and yeast cells and to some extend the case for carrageen (Irish Moss or Whirlfloc) and wort proteins. Other clarifiers like silica gel and tannic acid bind with proteins through reactions with their hydrophobic regions. Collagen is a very effective clarifier which is used after fermentation. It is the effective compound in isinglass and gelatin. collagen is a protein that, depending on its origin and processing has an isoelectic point of ~5.5 (isinglass) [Alton], 7.0 – 9.0 (type A gelatin) or 4.7-5.4 (type B gelatin) [GMIA]. Isinglass is produced from the swimbladder of tropical fish. Gelatin is either made from Type A gelatin is produces from pig skin and feet while type B gelatin is produced bovine (cow) parts. But for all of these sources of collagen their IEP (isoelectric point) is above the pH beer which means that the collagen molecules carry a net positive charge as we saw in Isoelectric point. Yeas cells however are negatively charged. After all, the actively pump protons, which carry a positive charge, into their environment. The different polatity in their electrical charges cause the yeast to be attracted to the collagen molecules. Due to is size the collagen slowly settles to the bottom of the vessel and takes the yeast with it. Gelatin and isinglass are not only known to aid yeast settlement but remove protein hazes as well. Proteins in beer are largely positively charged (the same charge as the collagen) and gelatin and isinglass will show little to no attracting to the protein part of beer hazes. But protein haze is formed by bonds between tannins and protein and it is the tannins that collagen bonds with. When it then settles to the bottom it takes the protein-tannin complexes, that cause the haze, with it. Because of its wide availability (almost every grocery store carries it), most brewers use Knox unflavored gelatin if they need to clarify a beer. Knox gelatin is made from pork and therefore type A [Kraftfoods]. Whenever I use it I have been using about 3-4 g (½ packet) dissolved in 100-200 ml warm water. The water should be sanitary and sanitary procedures should be followed since the gelatin suspension cannot be boiled. Once the gelatin is completely dissolved the mix is added to the beer and mixed in well. It should take only a few days for the gelatin to settle after which the clear beer can be drawn off. Clarifiers can also be used in the boil kettle. Popular products are Irish Moss and Whirlfloc. The active ingredient in both is carrageen. In contrast to collagen carraeen is a polysaccaride that is strongly negatively charged over the pH range encountered in brewing [Martin, 2000]. But at boil pH most proteins are at or slightly above their isoelectric point which means they carry a neutral or slightly negative charge and should therefore not be attracted to carrageen. In fact the use of carrageen (e.g. Irish Moss) in the boil does not increase the protein precipitation but they cause the formation of larger flocks that settle more quickly and form better trub cones in the whirlpool [Lewis&Bamforth, 2006]. The use of Irish Moss or Whirlflock is not permitted by the German purity law. Neither is gelatin, but isinglass can be used if the beer is subsequently filtered [Narziss, 2005]. the taste of the beerLast but not least the pH of the beer also affects its taste perception. A low beer pH results in a crisper more lively beer while a high beer pH is generally associated with a dull flavor perception. But there are limits to how low the pH can be before the beer's taste starts to take on sour notes. For all malt beers a pH range of 4.25 - 4.6 [Narziss, 2005] is generally accepted as optimal while adjunct beers can be as low as 4.0 [Kunze, 2007] and sour beers will be even lower. The lower pH of adjunct beers is a result of the lower buffer capacity that adjunct mashes have. The yeas is able to lower the pH further because the beer does not offer as much resistance as it does in all malt beers. How pH changes the taste becomes evident if the taste of a post boil wort sample and that of very young beer is compared. At the beginning of the fermentation the yeast lowers the pH while no or little fermentation has happened yet. As a result that sample will taste sweeter and fresher than the unpitched post boil sample which tastes more dull even though it has a nearly identical sugar profile.
References
|