At home water testing
|
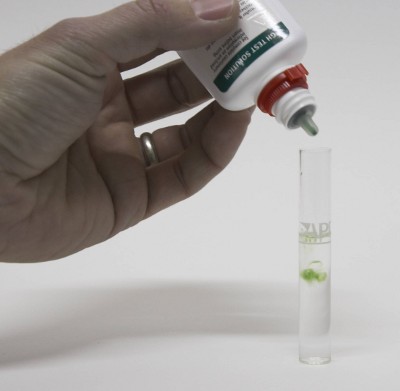
Water composition is important for brewing and many brewers either send their water to a lab for analysis for build their water from scratch by using very soft (e.g. reverse osmosis water) and salts. It is, however, also possible to test brewing water at home. The precision and amount of detail of such a water test at home does not match that of a professional analysis, but it is sufficient to estimate the residual alkalinity of the brewing water with an acceptable precision. At home water testing also allows regular testing of the water source in order to detect seasonal changes that may warrant a more precise professional analysis. Contents[hide]Test kits Figure 1 - A typical GH&KH test kit for aquarium use. It contains test tubes, test chemicals and instructions. The total price for kits like these is around $6 and while there are more expensive test kits available for fish owners they are useless to brewers since they test water parameters that aren't of interest for brewing. To do that water testing we brewers can use water test kits that are available for aquarium owners. The kind of test kit that we need is one that tests GH and KH. GH stands for General Hardness and measures the total hardness of the water. In water speak the total hardness is the amount of calcium and magnesium ions present. It is commonly measured as either dH (German Hardness) or ppm as CaCO3. Both these units are equivalent measures. That measns that they don't express the weight of the calcium and the magnesium ions but their number multiplied by their electrical charge. The latter is 2+ for both of them. With knowledge of the atomic weight and an guess on the calcium to magnesium ratio commonly found in water one can estimate the calcium and magnesium content of the analyzed water [DeLange]. KH stands for Karbonat Härte (German for carbonate hardness) which is the alkalinity of the water. Like total hardness or GH it is measured as either German Hardness (dH) or ppm as CaCO3. The conversion of 1 dH = 17.8 CaCO3 is true for both total hardness and alkalinity. Two types test kits are available: titration based and strips. Titration based test kits contain test tubes and chemicals that are added until there is a color reaction in the water. Test strips are submersed in the water and the color reaction on their test pads is then compared against a color scale. While more difficult to use I prefer titration based tests since they provide more precise results are are easier to "read" than strips. Test procedure | |
|
For the exact instructions how to use the test kit read the instruction that come with it. In particular how to convert the amount of titrant, that has been added, to a hardness value in dH or ppm as CaCO3. But in general they all work the same:
Titration |
|
|
As mentioned earlier the process by which these tests work commonly known as titration. During a titration a chemical, titrant, is added to a sample of known amount. This titrant reacts with the compound to be tested until all of that compound in the sample has been consumed. An indicator signals that this point has been reached. Based on the amount of titrant that has been added the unknown amount of the compound in the tested solution can be calculated. In the case of an alkalinity test (KH test) a strong acid is added that consumes the carbonates and bicarbonates which establish the water's alkalinity. That reaction produces carbonic acids and more importantly lowers the pH. Once the pH as been lowered to about 4.3 virtually all carbonates and bicarbonates have been converted. At that point the indicator solution, which is nothing more than a pH indicator changes color from blue to yellow/green. The concentration of the test solution is designed such that each drop contains enough acid to neutralize 1 dH or 17.8 ppm as CaCO3 in 5 ml water. As a result the precision of the test can be increased by increasing the sample size to 10 ml and assuming that each drop stands for 0.5 dH or 8.9 ppm as CaCO3. A similar reaction takes place during testing for GH.
|
|
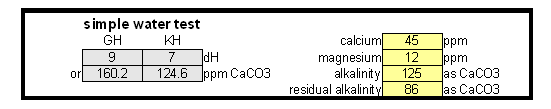
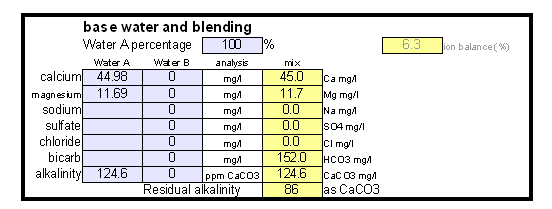
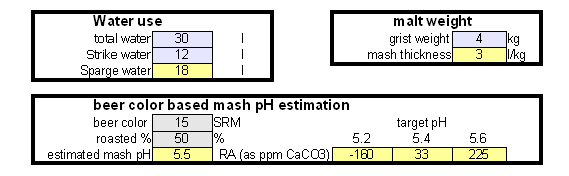
Estimating Residual Alkalinity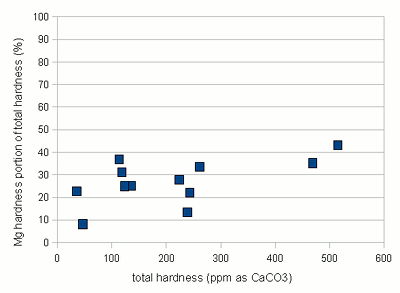 Figure 5 - Portion of magnesium hardness in percent plotted over the total hardness for a number of water profiles posted by members of the homebrewtalk.com on-line forum Just knowing alkalinity and total hardness of the water doesn't help for brewing. To calculate residual alkalinity, an important water characteristic that expresses the water's aklalinity that takes affect in brewing, calcium and magnesium concentrations need to be known. Total hardness, however, lumps them both together since calcium and magnesium are contributors. When looking at various water reports one will find that while the calcium to magnesium ratio is not constant about 30% of the total hardness is contributed by magnesium. This seems to be true for for most waters and Figure 5 illustrates that with data from various water profiles that brewers posted on-line (homebrewtalk.com). Using that relationship the residual alkalinity can be estimated as RA = KH - GH / 4 This formula works with KH and GH given as either dH or ppm as CaCO3. Using the test results in a spread sheetIf you don't want to calculate residual alkalinity by hand or want to estimate mash pH, you can use the water calculator. This is an Excel spread sheet that, among other features, allows entering KH and GH results from a simple water test. If you don't own Microsoft Excel check out OpenOffice which is a free office suite that supports excel spreadsheets. In fact, I develop all my spreadsheets with that software. Step 1: enter the GH and KH test restults. If not entered as ppm as CaCO3 the measurement is converted to that units. With the assumption that 30% of the total harndness are contributed by Magnesium the calcum and magnesium content of the water are calculated. No assumtion needs to be made to calculate the alkalinity. In this case the residual alkalinity is If no other values are given in the field for the more detailed water report, the results from the GH & KH test are carried over and will be used by the rest of the spreadsheet. Step 2: After strike water volume, grist weight and beer color have been specified an mash pH estimate is made based on these parameters and the residual alkalinity of the water. "Roasted %" refers specifies the percentage of specialty malts which are roasted malts (e.g. Roasted Barley, chocolate, Carafa, black patent and others).
|