Difference between revisions of "An Overview of pH"
(→pH buffers) |
(→pH buffers) |
||
| (73 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | {| style="width: | + | {| style="width:860px" |
| − | | | + | |- |
| + | |||
| + | [[Image:PH_overview_header.jpg]] | ||
| + | |||
| + | |- | ||
| + | | This is the first article in a three article series intended to be an overview of pH, the importance of pH in brewing, how pH affects various brewing processes and how it can be measured and controlled. It tries to keep mathematical equations and equations for chemical reactions to a minimum in order to make the subject understandable for a wider audience. | ||
| − | |||
| − | |||
= What is pH ? = | = What is pH ? = | ||
| − | + | |- | |
| + | | | ||
| − | + | pH is a measure of the acidity of a solution. It is a measure of the concentration of hydrogen ions (H<sup>+</sup>; proton). The higher the concentration of hydrogen ions in a solution the more acidc it is and the lower their concentration the more alkaline it is. In pure water (H<sub>2</sub>O) not all of the hydrogen (H) and oxygen (O) atoms are bound in water molecules. A small number of these molecules are broken up (disassociated) into protons (H<sup>+</sup>) and hydroxide (OH<sup>-</sup>) ions (see figure 1). The H<sup>+</sup> concentration of freshly distilled water, which is water that didn't have a chance to pick up any minerals or gases, corresponds to a pH of 7 which is the neutral pH. An acidic solution will have a pH below 7 and an alkaline solution will have a pH greater than 7. Once distilled water stands in air it will pick up CO<sub>2</sub> and the pH falls to about 6.5. | |
| − | + | | valign="top" | [[Image:Icon_basics.gif|link=|alt={Brewing Basics}]] | |
| + | |- | ||
| + | | | ||
| − | + | Technically free protons (H<sup>+</sup>) don't exist in water. They react with water molecules to form a hydronium ion (H<sub>3</sub>O<sup>+</sup>) but for the sake of simplicity in this article I will refer to them as protons, as all that matters is their concentration and their electrical charge [Wikipedia-1]. | |
| − | + | The pH scale is not a linear measure of the proton concentration but a logarithmic one. In fact, the “p” in “pH” stands for the negative base 10 logarithm and “H” stands for the concentration of H<sup>+</sup>. This means that a change of 1 pH unit doesn't change the proton concentration by 1/7 of that of distilled water. Instead a solution with a pH of 6 has a 10 times higher proton concentration compared to distilled water. And a pH of 5 means it has a 100 (i.e. 10x10) times higher proton concentration. Likewise a solution with a pH of 8 has only 1/10 the proton concentration of distilled water. For each pH unit up the concentration is divided by 10 and for each pH unit down it is multiplied by 10. | |
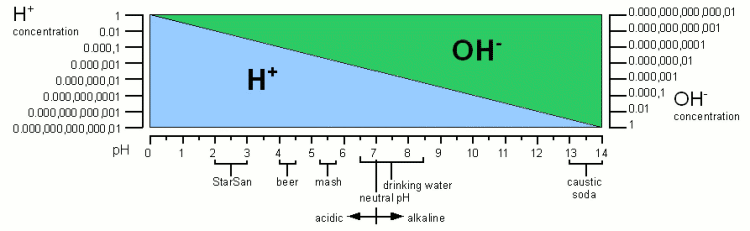
| − | + | [[Image:PH_ranges.gif|frame|center|'''Figure 1''' - The concentration of protons (H<sup>+</sup>) and hydroxide ions (OH<sup>-</sup>) over the 0-14 pH range. It also lists the pH of substances commonly encountered in brewing]] | |
| − | + | An important law regarding the concentration of H<sup>+</sup> and OH<sup>-</sup> is that the product of their concentrations has to remain constant. This means if OH<sup>-</sup> is added, through Sodium Hydroxide (NaOH) for example, the H<sup>+</sup> concentration has to fall. This explains how the pH rises when OH<sup>-</sup> is added even though pH is a measure of the H<sup>+</sup> concentration and not the OH<sup>-</sup>. If the concentrations for H<sup>+</sup> and OH<sup>-</sup> are written as negative logarithms (pH for H<sup>+</sup> and pOH for OH<sup>-</sup>) their relationship is simply pH + pOH = 14. | |
| − | + | |- | |
| + | | | ||
| − | Later we will see how | + | = Weak acids and bases = |
| + | |||
| + | [[Image:Acetic_acid_disassociation.gif|right|frame|'''Figure 2 - The behavior of a weak acid at different pH.''' The relationship between the percentage of an acid (acetic acid (vinegar), HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>) and its conjugate base (acetate, C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup>). The pH at which the concentration of acid and its conjugate base are equal is called pKa is an equilibrium constant between the acid and its conjugate base and it depends on the type of acid and the temperature. For acetic acid at 20 C (68 F) it is 4.75. Below that pH an increasing amount of acetate anions will absorb protons and become acetic acid. Above that pH an increasing amount of acetic acid will lose protons and become acetate anions. Or to express it another way, in order to decrease the pH protons have to be added to convert acetate anions into acetic acid.]] | ||
| + | |||
| + | When we later discuss the effects of pH we will come across one common theme: the disassociation of H<SUP>+</SUP> and OH<SUP>-</SUP> ions from larger molecules. A substance that donates H<SUP>+</SUP> to or accepts OH<SUP>-</SUP> ions from its environment is called an acid. It lowers the pH. A substance that accepts H<SUP>+</SUP> or donates OH<SUP>-</SUP> is called a base and it raises the pH. When acids and bases are brought together they neutralize each other by forming a salt and water. | ||
| + | |||
| + | When strong acids or bases are dissolved in water they readily dissolve and each molecule donates H<SUP>+</SUP> or OH<SUP>-</SUP>. Depending on the concentration of the acid or base the result is a dramatic pH drop towards 0 or a pH rise towards 14. That's why they are used for titration (will be explained later): the number of added protons (H<SUP>+</SUP>) or hydroxide ions (OH<SUP>-</SUP>) depends only on the amount of strong acid or strong base that is added and not on the pH of the environment. But most acids and bases we will deal with in brewing, especially when we talk about the effects of pH, are weak acids. A weak acid is an acid where only a portion of its molecules disassociate in water. The exact ratio depends on the pH of the solution, temperature and the type of acid. The same is true for weak bases. | ||
| + | |||
| + | When an acid dissociates it leaves behind a negatively charged remainder, called a conjugate base. It is called a base because once it has lost one or more protons it can accept them again, which is what bases do. Since the ratio between an acid and its conjugate base depends on pH, pH controls how many negatively charged conjugate bases are present. In case of weak bases the remainder (called a conjugate acid, because it can accept OH<SUP>-</SUP> or donate H+) that is left after the dissociation of a hydroxide ion (OH<SUP>-</SUP>) or the acceptance of a proton is positively charged. pH controls their ratio as well. Figure 2 illustrates the pH dependent ratio of acid and conjugate base on the example of acetic acid (vinegar). | ||
| + | |||
| + | Later we will see how weak acids such as tannins and complex molecules build from many different weak acids and weak bases (proteins for example) are affected by the pH of their environment though the pH dependent disassociation of H<SUP>+</SUP> and OH<SUP>-</SUP>. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_basics.gif|link=|alt={Brewing Basics}]] | ||
| + | |- | ||
| + | | | ||
= pH buffers = | = pH buffers = | ||
| − | + | [[Image:Vinegar_titration.gif|frame|right|'''Figure 3 - Titration of vinegar.''' A strong base (sodium hydroxide, NaOH, a.k.a. lye or caustic soda) is added to 100 ml of 3 different concentrations of acetic acid (vinegar). The pH of a pure acetic acid solution depends on its concentration and is shown on the left. As the base is added it converts more and more of the acetic acid to acetate. The ratio of acetate and acetic acid determines the pH according to Figure 2. The acetic acid and acetate together acts as a buffer and the pH changes little with increasing additions of sodium hydroxide. At point B (in the case of the 5% acetic acid solution) 50% of the acetic acid has been converted to acetate and its pKa of 4.75 is reached. At point A all of the acetic acid has been converted and the pH shoots up. The buffering capacity of the acetic acid/acetate buffer has been consumed by the sodium hydroxide and now the sodium hydroxide controls the pH. Since it is a strong base it quickly raises the pH towards 14. The process of adding controlled amounts of acid or bases to a sample is called titration and can be used to determine the buffer capacity of that sample.]] | |
| − | + | [[Image:Buffer_analogy.gif|frame|right|'''Figure 4 - a mechanical analogy to chemical buffers''' The acids and bases in a solution like are the springs in this model. In both A and C the springs hold the needle in place at the same marks on the scale. This is like 2 solutions having the same pH held in place by its acids and bases. But the strength with which the needle is held in place becomes only apparent when another spring is added which compares to adding more acid or base to a solution. In the weakly buffered model (B) the needle moves considerably while in the strongly buffered model (D) it moves hardly at all. The limitation of this analogy is, that adding more springs will never completely "consume" the opposing springs. Unlike during the titration of a buffered solution there will never be a point at which it takes much less force to move the needle after enough springs have been added]] | |
| − | + | Another fundamental pH related chemistry phenomenon is pH buffers and their buffer capacity. | |
| − | + | pH only tells us about the balance between protons (H<SUP>+</SUP>) and hydroxide ions (OH<SUP>-</SUP>) in a solution. It doesn't tell us how strongly it is held in place, i.e. it may take the addition of only little amounts of an acid or a base to change the pH or it may take a lot to change it. What is keeping the pH in place are weak acids, bases and their salts. They all act act as ''pH buffers''. In computers and elsewhere buffers are systems which are designed to absorb shocks and sudden changes. pH buffers are solutions that can resist sudden or gradual pH changes, to some extend as we will see, brought on by the addition of acids or bases. | |
| − | + | We saw in the last section that the ratio between acid and conjugate base molecules and base and conjugate acid molecules depends on the pH and the type of acid or base itself. Let’s look at a solution of acetic acid and acetate. At a pH of 4.75 half of the molecules are still acetic acid and the other half is its conjugate base acetate. When we now try to lower the pH by adding more H<SUP>+</SUP> through the addition of a strong(er) acid the ratio of acetic acid and acetate molecules shifts towards acetic acid (Figure 2). This means that some, if not the majority, of the H<SUP>+</SUP> that is added will be consumed by creating more acetic acid molecules from the existing acetate molecules. This in turn reduces the number of protons that are available to lower the pH and as a result the pH doesn't fall as much as it would have if the acetic acid was not present or present at a lower concentration. In other words, the vast majority of H<sup>+</sup> added goes towards shifting the ratio of acetic acid and acetate and only very little is used to actually change the H<sup>+</sup> concentration. The acetic acid and acetate mix acted as a buffer. The amount of stronger acid that needs to be added to lower the pH depends not only on the desired pH difference but also on the concentration of acetic acid and acetate. The higher their concentration the more acid it takes to lower the pH and the stronger the buffering capacity of the solution is. | |
| − | + | This also works in the other direction when a strong base is added. In this case the rise in pH requires that more acetic acid is converted into acetate. This liberates protons which fight the pH rise. This is illustrated in Figure 3. | |
| − | + | In mashing and in beer there is not a single acid or base that acts as a buffer, but rather a wide variety which have different pKa values. The combined effect of all these buffer systems determines the pH of the wort or beer. And later when we talk adjusting mash pH we will come back to the pH buffers that malt provides and how their amount determines how much acid it takes to move the mash pH into a favorable range for mashing. | |
| − | + | Buffer solutions also provide a stable pH reference for the calibration of pH meters. These solutions have been mixed precisely from acids/bases and their salts to hold their pH steady even in the presence of small contamination with other acids or bases. Contamination of such buffers is reduced by rinsing the pH meter electrode with soft or distilled water which has very little pH buffering capacity and is therefore not able to affect the pH of the buffer solution in any significant amount. | |
| − | [[Image:Isoelectric_point.gif|frame|center]] | + | You may also read [[A simple Model for pH Buffers]] for another demonstration on how pH buffers work. |
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
| + | |||
| + | =Isoelectric point (IEP)= | ||
| + | |||
| + | So far we have seen how pH controls the percentage of weak acids and weak bases that lose protons or accept them respectively. This creates a charged molecule. Many complex molecules (amino acids and proteins for example) contain a number of groups that can lose or accept protons depending on pH. The overall electrical charge of the molecule is the sum of all the charged groups. If there are acidic and basic groups on the molecule, i.e. groups that can become negatively charged (acidic) and groups that can become positively charged (basic) there will be a pH at which the sum of the electrical charges is zero and the molecule is electrically neutral. This pH is called the isoelectic point (IEP or pI) and it depends on the pH characteristics of the molecule's acidic and basic groups. | ||
| + | |||
| + | Figure 5 illustrates the IEP on Glutamic acid, one of the different amino acids that are the building block of peptides and proteins. | ||
| + | |||
| + | [[Image:Isoelectric_point.gif|frame|center|'''Figure 5 - The isoelectric point of Glutamic acid'''. Glutamic acid has 3 groups that can lose or accept proton and therefore change their electrical charge depending on pH. The relative concentration of the charged state of these groups is shown with the green, blue and red graphs. The yellow graph is the total charge of the amino acid. At pH 2.8, its isoelectric point, this charge is 0. (pKa data for Glutamic acid was taken from [Champe, 2007])]] | ||
| + | |||
| + | We will see later how the IEP and the pH dependence of electrical charges of various substances are of importance in brewing. | ||
| + | |||
| + | At a pH below its IEP the net charge of a molecule is always positive because the higher concentration of H<sup>+</sup> leads to more acceptance of H<sup>+</sup> by the molecule's acidic and basic groups. The opposite is true at a pH above its IEP where the low H<sup>+</sup> concentration causes the basic and acidic groups to donate H<sup>+</sup> to the environment which leaves the net charge on the molecule to be negative. | ||
| + | |||
| + | Some readers may wonder how the electrical charge of a molecule can change while the solution it is in is still electically neutral. The answer is that the balance is provided by cations (Na<sup>+</sup>, Ca<sup>2+</sup>, etc.), anions (SO<sub>4</sub><sup>2-</sup>, HCO<sub>3</sub><sup>-</sup>, etc) and other charged molecules which are necessary to hold the pH at that level. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
= Measuring pH = | = Measuring pH = | ||
| − | Now that we looked at how pH | + | Now that we looked at the chemical affects that are important for understanding pH lets have a look at how pH can be measured. For the home brewer 3 viable methods of testing pH exists: economy pH paper, colorpHast strips and pH meters. In general the cheaper the means of pH testing the less accurate the result is. |
| − | + | |- | |
| + | | | ||
| − | + | ==pH indicator solutions== | |
| − | + | [[Image:Red_cabbage_pH_series.jpg|frame|right|Figure 6 - Red cabbage juice as pH indicator. While this is a neat science experiment, the pH sensitivity of red cabbage juice is too broad to be useful for pH measurements in brewing.]] | |
| − | + | pH indicators are weak acids or bases, they dissociate into a conjugate base or conjugate acid depending on pH. For pH indicators the acid and the conjugate base(s) (acid which lost one or more protons) have different colors. Because the relative concentrations of these two or more forms change with pH (see Figure 2) the color of the indicator also changes with pH. A number of different indicator substances have been discovered each of which works best in its own pH range [Wikipedia-2]. | |
| − | A | + | A simple pH indicator can be made from red cabbage juice and its color for different pH is shown in Figure 6. While this is great for illustrating pH indicators it has little practicality in brewing since the precision of pH readings is too low to be useful. The pH indicator in the red cabbage juice (Anthocyanin) can actually lose more than one proton depending on the pH of the environment. The number of protons that a given molecule lost determines its color and as a result we see the color change from read to blue and then to yellow. |
| + | |||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
| + | |||
| + | ==Litmus paper and economy pH strips== | ||
| + | |||
| + | When the aforementioned pH indicator solution is applied to filter paper and dried, simple pH indicator strips can be made. These strips, sometimes called litmus paper, are the cheapest means of testing the pH of a solution. | ||
| + | |||
| + | To test the pH of a sample a strip is dipped into the sample or a few drops of the sample are placed onto the test paper. After removing it from the sample the color reaction is compared to a color scale that is supplied with the strips. The pH value shown for the color that matches the strip's color reaction is the approximate pH of the sample. | ||
| + | |||
| + | Since the indicators themselves act as acids or bases they actually change the pH of the tested solution if it is not sufficiently buffered. It is important to think about the buffering capacity of the solution that is tested for pH with any of the means of testing pH. The less its buffering capacity the less accurate the pH measurement will be. Luckily most solutions that we test for pH in brewing are strongly buffered and we don't have to worry about this effect. The only one that is not strongly buffered is very soft or even distilled water. Here you can easily get false readings but the pH of the brewing water is of little importance anyway, especially when buffered only weakly, as we will see later. | ||
| + | |||
| + | A good analogy to testing the pH of weakly and strongly buffered solutions is measuring the temperature of a small or large sample. If the sample is small the temperature of the probe will change its temperature because as the sample and the probe reach temperature equilibrium the temperature of the sample has to change. This obviously doesn't cause a significant error if the sample size is much larger than the temperature probe. The same applies to testing pH and the buffering capacity of the sample. | ||
==colorpHast strips== | ==colorpHast strips== | ||
| − | EMD chemicals | + | [[Image:Colorphast.jpg|right|frame|'''Figure 7 - colorpHast precision pH test strips''' [northernbrewer.com]]] |
| + | |||
| + | EMD chemicals make a range of pH test strips that are a significant improvement over litmus paper or other economic pH test strips. The product is called colorpHast and has these advantages: | ||
* the indicator will not run and leach out of the test strip | * the indicator will not run and leach out of the test strip | ||
| − | * the testing range is narrow enough to read the strips with +/- 0.2 pH | + | * the testing range is narrow enough to read the strips with +/- 0.2 pH unit precision |
* the strips are easily matched against the color scale | * the strips are easily matched against the color scale | ||
| − | * they are designed to be used in samples that contain proteins and don't exhibit a protein error [ | + | * they are designed to be used in samples that contain proteins and don't exhibit a protein error [EMD] |
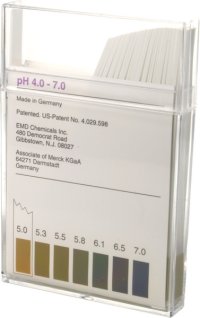
| − | Another important advantage is that they don't require calibration like a pH meter does. But their precision is limited to ~+/- 0.2 pH unit which is sufficient for testing the mash pH but some brewers like to have greater precision in their pH measurements. In comparative tests between the colorpHast strips and a pH meter | + | The type most suitable for brewing is the strips with a pH range of 4.0 - 7.0 (see Figure 7). |
| + | |||
| + | Another important advantage is that they don't require calibration like a pH meter does. But their precision is limited to ~+/- 0.2 pH unit which is sufficient for testing the mash pH but some brewers like to have greater precision in their pH measurements. In comparative tests between the colorpHast strips and a pH meter a constant error of about 0.3 pH units was noticed when they are used in the 5.0 - 6.0 pH range; i.e. the strips read about 0.3 pH units lower than the pH that was determined with a pH meter. More info on the precision of the colorpHast strips can be found here: | ||
| + | * [[colorpHast_vs_pH_meter|colorpHastStrips vs. a pH meter]] | ||
| + | * [[Effects_of_mash_parameters_on_fermentability_and_efficiency_in_single_infusion_mashing#pH_meter_vs._colorpHast_strips| micro mashing experiments]] | ||
| + | * [[An Evaluation of the suitability of colorpHast strips for pH measurements in home brewing]] | ||
| + | |||
| + | |- | ||
| + | | | ||
==pH meters== | ==pH meters== | ||
| − | A | + | [[Image:PH_electrode.gif|frame|right|'''Figure 8 - schematic drawing of a pH meter''' A pH meter consists of 3 parts: a pH probe that converts the pH of the solution into an electrical potential, an amplifier and analog digital converter that converts that electrical potential into a pH reading that is then shown on the display. The heart of the pH meter is the glass bulb at the tip of the pH probe. It is coated on both sides with an ion selective hydrated gel which acts like a weak acid [ktf-split.hr], i.e. the pH of the surrounding medium determines how many of its molecules lost their protons (H<sup>+</sup>) (see figure 2). The pH inside the bulb, and with it the level of deprotonation, is fixed through a solution of hydrochloric acid (HCl). But the pH to which the outside layer is exposed is not fixed and it determines the level of deprotonation of that layer. If it is different from the inside layer an electrical potential is created which is picked up with the 2 electrodes. The stronger the pH difference the greater this electrical potential will be. The potential is then amplified and converted to a pH reading with the help of calibration settings. This pH value is then shown on the display.]] |
| − | A | + | A completely different approach for taking pH readings is the use of an electronic pH meter. The heart of these meters is a glass electrode which converts the H<sup>+</sup> concentration into a voltage that can be measured with a voltmeter. Since this voltage is proportional to the logarithm of the H<sup>+</sup> concentration it is also proportional to the pH; in other words the pH of the sample is a linear function of the voltage that is measured at the probe. Every linear function is determined by 2 parameters: offset and slope. The slope can be calculated and is about 60 mV per pH unit [Wikipedia-3] but can change as the probe ages. |
| − | + | A single point calibration uses a single solution of known pH. This solution is called a calibration buffer and the pH for common buffers are 4.00, 7.00 and 10.00. Since such a single point calibration can only determine the current offset of the probe a better calibration procedure uses 2 buffers. Most brewing related samples have a pH below 7 and calibrating the pH meter with 4.00 and 7.00 pH buffers is recommended. This calibration should be carried out regularly. Especially when the probe has not been used for a while. | |
| − | + | When the pH of a sample is measured it is also important to know the temperature of the sample. This is important for 2 reasons. The temperature of the sample affects its H<SUP>+</SUP> concentration and therefore its pH. This is the result of changing H<SUP>+</SUP> and OH<SUP>-</SUP> dissociation balances in the sample and is substrate specific. For wort it has been reported that the pH at mash temperatures (65 C/150 F) is about 0.35 pH units lower than at room temperature and at mash out temperatures (75 C/170 F) it is even 0.45 pH units lower [Briggs, 2004]. While my own experiments showed only a ~0.2 pH difference between a sample at mash temperature and a sample at room temperature (25 C/77 F). The temperature dependent change of pH is also important for the calibration of the pH meter since the buffer solutions have their nominal pH only at a specific temperature. This temperature is usually room temperature (25 C/77 F) and many buffer manufacturers supply tables with their buffers that show how their pH changes with temperature. I have made it a habit to always calibrate my pH meter with buffer solutions at room temperature (25 +/- 1 C). | |
| − | If the pH is also dependent on temperature what temperature should a sample be measured | + | In addition to the pH of the sample the temperature also affects the response of the pH electrode. This effect is specific to the electrode and will not change from sample to sample that's why many pH meters are equipped with automatic temperature correction (ATC) where a separate temperature sensor, which in less expensive meters is oftentimes integrated into the probe, is used to measure the temperature of the sample and used to correct for the temperature error of the pH electrode. This means that the pH measured with an ATC pH meter still changes based on temperature but that change is only the result of temperature dependent H<sup>+</sup> concentration in the sample. It is a true pH change in the sample. |
| + | |||
| + | If the pH is also dependent on temperature what temperature should a sample be measured? The answer is that any temperature works as long as it is noted and reported with the pH measurement. To make comparing of pH measurements easier it is best to take them at a standard temperature; 25 C/77 F or 20 C/68 F are common. Some brewers use their pH meter to test the pH directly in the mash. But testing hot liquids reduces the lifetime of the probe which is why I don't recommend this practice. We will come back to this topic when we look at the target pH for mashing and boiling. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
The increased precision of a pH meter comes at a price. Not only are they more expensive, the probes need replacement after 1-2 years. Good care can extend the life of the probe. Here are some tips when working with pH meters: | The increased precision of a pH meter comes at a price. Not only are they more expensive, the probes need replacement after 1-2 years. Good care can extend the life of the probe. Here are some tips when working with pH meters: | ||
| − | + | * Always store the probe in pH meter storage solution recommended by the manufacturer and never in water even it it is distilled water. A pH meter storage solution contains salts that help the pH meter recover. | |
| − | + | * Never let the probe dry out. | |
| − | + | * Thoroughly rinse the probe with distilled or deionized water, after removing from the storage solution, between measuring samples or calibration buffers and before returning to the storage solution. A quick flick with the wrist also removes the excess water from the probe. | |
| − | + | * Measure cooled samples only. | |
| − | + | * Move the probe around in the sample. This avoids localized pH changes caused by the acidic nature of the probe. | |
| + | * Don't scratch or rub the glass bulb. It is coated with a sensitive layer that is integral to its function and it is rather fragile. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| + | |- | ||
| + | | | ||
| + | |||
| + | A short buying guide for pH meters can be found here: [[pH Meter Buying Guide]] | ||
| + | |||
| + | =References= | ||
| + | |||
| + | :[Wikipedia-1] [http://en.wikipedia.org/wiki/Hydronium Hydronium] | ||
| + | :[Champe, 2007] Pamela C. Champe, Richard A. Harvey, Denise R. Ferrier, Lippincott's Illustrated Reviews: Biochemistry, 2007 | ||
| + | : [Wikipedia-2] [http://en.wikipedia.org/wiki/PH_indicator pH indicator] | ||
| + | :[EMD] [http://www.emdchemicals.com/analytics/literature/colorpHast_Products_for_pH_Testing.pdf colorpHast data sheet], EMD Chemicals, | ||
| + | :[ktf-split.hr] [http://www.ktf-split.hr/glossary/en_o.php?def=glass%20electrode, glass electrode], www.ktf-split.hr | ||
| + | :[Wikipedia-3] [http://en.wikipedia.org/wiki/PH_meter pH meter] | ||
| + | :[Briggs, 2004] Dennis E. Briggs, Chris A. Boulton, Peter A. Brookes, Roger Stevens, Brewing Science and Practice, Published by Woodhead Publishing, 2004 | ||
| + | |||
| + | =Acknowledgements= | ||
| + | |||
| + | I'd like to thank the members of the [http://forum.northernbrewer.com/ Northern Brewer] and [http://www.homebrewtalk.com/ HomeBrewTalk.com] forums that helped with reviewing and proof reading the article. And special thanks to analytical chemistry professor J. Schneider who took time to review the article and give it his professional blessing. | ||
| + | |||
| + | '''Next:''' [[How pH affects brewing]] | ||
|} | |} | ||
Latest revision as of 02:57, 16 March 2011
| This is the first article in a three article series intended to be an overview of pH, the importance of pH in brewing, how pH affects various brewing processes and how it can be measured and controlled. It tries to keep mathematical equations and equations for chemical reactions to a minimum in order to make the subject understandable for a wider audience.
ContentsWhat is pH ? | |
|
pH is a measure of the acidity of a solution. It is a measure of the concentration of hydrogen ions (H+; proton). The higher the concentration of hydrogen ions in a solution the more acidc it is and the lower their concentration the more alkaline it is. In pure water (H2O) not all of the hydrogen (H) and oxygen (O) atoms are bound in water molecules. A small number of these molecules are broken up (disassociated) into protons (H+) and hydroxide (OH-) ions (see figure 1). The H+ concentration of freshly distilled water, which is water that didn't have a chance to pick up any minerals or gases, corresponds to a pH of 7 which is the neutral pH. An acidic solution will have a pH below 7 and an alkaline solution will have a pH greater than 7. Once distilled water stands in air it will pick up CO2 and the pH falls to about 6.5. |
|
|
Technically free protons (H+) don't exist in water. They react with water molecules to form a hydronium ion (H3O+) but for the sake of simplicity in this article I will refer to them as protons, as all that matters is their concentration and their electrical charge [Wikipedia-1]. The pH scale is not a linear measure of the proton concentration but a logarithmic one. In fact, the “p” in “pH” stands for the negative base 10 logarithm and “H” stands for the concentration of H+. This means that a change of 1 pH unit doesn't change the proton concentration by 1/7 of that of distilled water. Instead a solution with a pH of 6 has a 10 times higher proton concentration compared to distilled water. And a pH of 5 means it has a 100 (i.e. 10x10) times higher proton concentration. Likewise a solution with a pH of 8 has only 1/10 the proton concentration of distilled water. For each pH unit up the concentration is divided by 10 and for each pH unit down it is multiplied by 10. An important law regarding the concentration of H+ and OH- is that the product of their concentrations has to remain constant. This means if OH- is added, through Sodium Hydroxide (NaOH) for example, the H+ concentration has to fall. This explains how the pH rises when OH- is added even though pH is a measure of the H+ concentration and not the OH-. If the concentrations for H+ and OH- are written as negative logarithms (pH for H+ and pOH for OH-) their relationship is simply pH + pOH = 14. | |
Weak acids and bases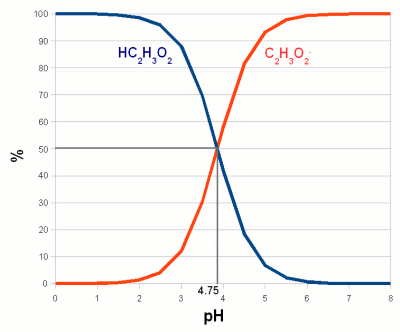 Figure 2 - The behavior of a weak acid at different pH. The relationship between the percentage of an acid (acetic acid (vinegar), HC2H3O2) and its conjugate base (acetate, C2H3O2-). The pH at which the concentration of acid and its conjugate base are equal is called pKa is an equilibrium constant between the acid and its conjugate base and it depends on the type of acid and the temperature. For acetic acid at 20 C (68 F) it is 4.75. Below that pH an increasing amount of acetate anions will absorb protons and become acetic acid. Above that pH an increasing amount of acetic acid will lose protons and become acetate anions. Or to express it another way, in order to decrease the pH protons have to be added to convert acetate anions into acetic acid. When we later discuss the effects of pH we will come across one common theme: the disassociation of H+ and OH- ions from larger molecules. A substance that donates H+ to or accepts OH- ions from its environment is called an acid. It lowers the pH. A substance that accepts H+ or donates OH- is called a base and it raises the pH. When acids and bases are brought together they neutralize each other by forming a salt and water. When strong acids or bases are dissolved in water they readily dissolve and each molecule donates H+ or OH-. Depending on the concentration of the acid or base the result is a dramatic pH drop towards 0 or a pH rise towards 14. That's why they are used for titration (will be explained later): the number of added protons (H+) or hydroxide ions (OH-) depends only on the amount of strong acid or strong base that is added and not on the pH of the environment. But most acids and bases we will deal with in brewing, especially when we talk about the effects of pH, are weak acids. A weak acid is an acid where only a portion of its molecules disassociate in water. The exact ratio depends on the pH of the solution, temperature and the type of acid. The same is true for weak bases. When an acid dissociates it leaves behind a negatively charged remainder, called a conjugate base. It is called a base because once it has lost one or more protons it can accept them again, which is what bases do. Since the ratio between an acid and its conjugate base depends on pH, pH controls how many negatively charged conjugate bases are present. In case of weak bases the remainder (called a conjugate acid, because it can accept OH- or donate H+) that is left after the dissociation of a hydroxide ion (OH-) or the acceptance of a proton is positively charged. pH controls their ratio as well. Figure 2 illustrates the pH dependent ratio of acid and conjugate base on the example of acetic acid (vinegar). Later we will see how weak acids such as tannins and complex molecules build from many different weak acids and weak bases (proteins for example) are affected by the pH of their environment though the pH dependent disassociation of H+ and OH-. |
|
pH buffers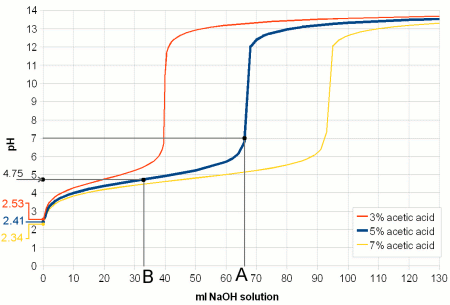 Figure 3 - Titration of vinegar. A strong base (sodium hydroxide, NaOH, a.k.a. lye or caustic soda) is added to 100 ml of 3 different concentrations of acetic acid (vinegar). The pH of a pure acetic acid solution depends on its concentration and is shown on the left. As the base is added it converts more and more of the acetic acid to acetate. The ratio of acetate and acetic acid determines the pH according to Figure 2. The acetic acid and acetate together acts as a buffer and the pH changes little with increasing additions of sodium hydroxide. At point B (in the case of the 5% acetic acid solution) 50% of the acetic acid has been converted to acetate and its pKa of 4.75 is reached. At point A all of the acetic acid has been converted and the pH shoots up. The buffering capacity of the acetic acid/acetate buffer has been consumed by the sodium hydroxide and now the sodium hydroxide controls the pH. Since it is a strong base it quickly raises the pH towards 14. The process of adding controlled amounts of acid or bases to a sample is called titration and can be used to determine the buffer capacity of that sample. 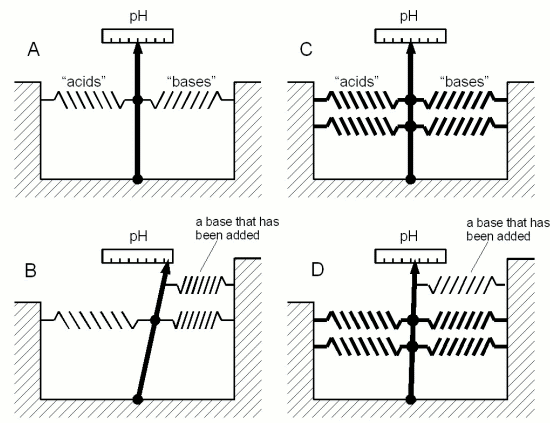 Figure 4 - a mechanical analogy to chemical buffers The acids and bases in a solution like are the springs in this model. In both A and C the springs hold the needle in place at the same marks on the scale. This is like 2 solutions having the same pH held in place by its acids and bases. But the strength with which the needle is held in place becomes only apparent when another spring is added which compares to adding more acid or base to a solution. In the weakly buffered model (B) the needle moves considerably while in the strongly buffered model (D) it moves hardly at all. The limitation of this analogy is, that adding more springs will never completely "consume" the opposing springs. Unlike during the titration of a buffered solution there will never be a point at which it takes much less force to move the needle after enough springs have been added Another fundamental pH related chemistry phenomenon is pH buffers and their buffer capacity. pH only tells us about the balance between protons (H+) and hydroxide ions (OH-) in a solution. It doesn't tell us how strongly it is held in place, i.e. it may take the addition of only little amounts of an acid or a base to change the pH or it may take a lot to change it. What is keeping the pH in place are weak acids, bases and their salts. They all act act as pH buffers. In computers and elsewhere buffers are systems which are designed to absorb shocks and sudden changes. pH buffers are solutions that can resist sudden or gradual pH changes, to some extend as we will see, brought on by the addition of acids or bases. We saw in the last section that the ratio between acid and conjugate base molecules and base and conjugate acid molecules depends on the pH and the type of acid or base itself. Let’s look at a solution of acetic acid and acetate. At a pH of 4.75 half of the molecules are still acetic acid and the other half is its conjugate base acetate. When we now try to lower the pH by adding more H+ through the addition of a strong(er) acid the ratio of acetic acid and acetate molecules shifts towards acetic acid (Figure 2). This means that some, if not the majority, of the H+ that is added will be consumed by creating more acetic acid molecules from the existing acetate molecules. This in turn reduces the number of protons that are available to lower the pH and as a result the pH doesn't fall as much as it would have if the acetic acid was not present or present at a lower concentration. In other words, the vast majority of H+ added goes towards shifting the ratio of acetic acid and acetate and only very little is used to actually change the H+ concentration. The acetic acid and acetate mix acted as a buffer. The amount of stronger acid that needs to be added to lower the pH depends not only on the desired pH difference but also on the concentration of acetic acid and acetate. The higher their concentration the more acid it takes to lower the pH and the stronger the buffering capacity of the solution is. This also works in the other direction when a strong base is added. In this case the rise in pH requires that more acetic acid is converted into acetate. This liberates protons which fight the pH rise. This is illustrated in Figure 3. In mashing and in beer there is not a single acid or base that acts as a buffer, but rather a wide variety which have different pKa values. The combined effect of all these buffer systems determines the pH of the wort or beer. And later when we talk adjusting mash pH we will come back to the pH buffers that malt provides and how their amount determines how much acid it takes to move the mash pH into a favorable range for mashing. Buffer solutions also provide a stable pH reference for the calibration of pH meters. These solutions have been mixed precisely from acids/bases and their salts to hold their pH steady even in the presence of small contamination with other acids or bases. Contamination of such buffers is reduced by rinsing the pH meter electrode with soft or distilled water which has very little pH buffering capacity and is therefore not able to affect the pH of the buffer solution in any significant amount. You may also read A simple Model for pH Buffers for another demonstration on how pH buffers work. |
|
Isoelectric point (IEP)So far we have seen how pH controls the percentage of weak acids and weak bases that lose protons or accept them respectively. This creates a charged molecule. Many complex molecules (amino acids and proteins for example) contain a number of groups that can lose or accept protons depending on pH. The overall electrical charge of the molecule is the sum of all the charged groups. If there are acidic and basic groups on the molecule, i.e. groups that can become negatively charged (acidic) and groups that can become positively charged (basic) there will be a pH at which the sum of the electrical charges is zero and the molecule is electrically neutral. This pH is called the isoelectic point (IEP or pI) and it depends on the pH characteristics of the molecule's acidic and basic groups. Figure 5 illustrates the IEP on Glutamic acid, one of the different amino acids that are the building block of peptides and proteins. 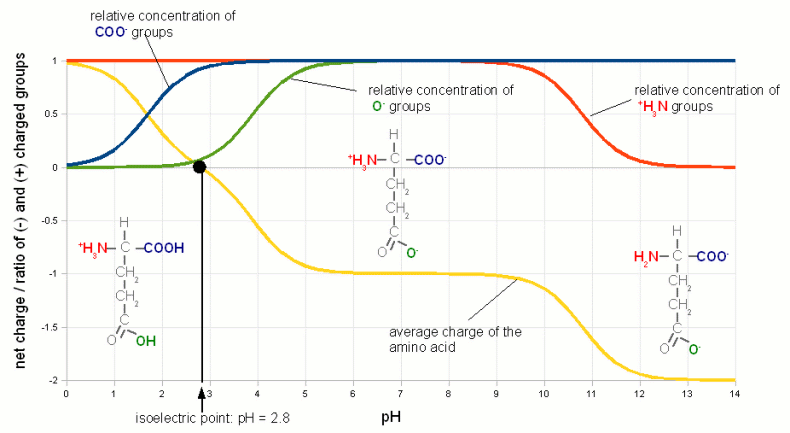 Figure 5 - The isoelectric point of Glutamic acid. Glutamic acid has 3 groups that can lose or accept proton and therefore change their electrical charge depending on pH. The relative concentration of the charged state of these groups is shown with the green, blue and red graphs. The yellow graph is the total charge of the amino acid. At pH 2.8, its isoelectric point, this charge is 0. (pKa data for Glutamic acid was taken from [Champe, 2007]) We will see later how the IEP and the pH dependence of electrical charges of various substances are of importance in brewing. At a pH below its IEP the net charge of a molecule is always positive because the higher concentration of H+ leads to more acceptance of H+ by the molecule's acidic and basic groups. The opposite is true at a pH above its IEP where the low H+ concentration causes the basic and acidic groups to donate H+ to the environment which leaves the net charge on the molecule to be negative. Some readers may wonder how the electrical charge of a molecule can change while the solution it is in is still electically neutral. The answer is that the balance is provided by cations (Na+, Ca2+, etc.), anions (SO42-, HCO3-, etc) and other charged molecules which are necessary to hold the pH at that level. |
|
Measuring pHNow that we looked at the chemical affects that are important for understanding pH lets have a look at how pH can be measured. For the home brewer 3 viable methods of testing pH exists: economy pH paper, colorpHast strips and pH meters. In general the cheaper the means of pH testing the less accurate the result is. | |
pH indicator solutionspH indicators are weak acids or bases, they dissociate into a conjugate base or conjugate acid depending on pH. For pH indicators the acid and the conjugate base(s) (acid which lost one or more protons) have different colors. Because the relative concentrations of these two or more forms change with pH (see Figure 2) the color of the indicator also changes with pH. A number of different indicator substances have been discovered each of which works best in its own pH range [Wikipedia-2]. A simple pH indicator can be made from red cabbage juice and its color for different pH is shown in Figure 6. While this is great for illustrating pH indicators it has little practicality in brewing since the precision of pH readings is too low to be useful. The pH indicator in the red cabbage juice (Anthocyanin) can actually lose more than one proton depending on the pH of the environment. The number of protons that a given molecule lost determines its color and as a result we see the color change from read to blue and then to yellow. |
|
Litmus paper and economy pH stripsWhen the aforementioned pH indicator solution is applied to filter paper and dried, simple pH indicator strips can be made. These strips, sometimes called litmus paper, are the cheapest means of testing the pH of a solution. To test the pH of a sample a strip is dipped into the sample or a few drops of the sample are placed onto the test paper. After removing it from the sample the color reaction is compared to a color scale that is supplied with the strips. The pH value shown for the color that matches the strip's color reaction is the approximate pH of the sample. Since the indicators themselves act as acids or bases they actually change the pH of the tested solution if it is not sufficiently buffered. It is important to think about the buffering capacity of the solution that is tested for pH with any of the means of testing pH. The less its buffering capacity the less accurate the pH measurement will be. Luckily most solutions that we test for pH in brewing are strongly buffered and we don't have to worry about this effect. The only one that is not strongly buffered is very soft or even distilled water. Here you can easily get false readings but the pH of the brewing water is of little importance anyway, especially when buffered only weakly, as we will see later. A good analogy to testing the pH of weakly and strongly buffered solutions is measuring the temperature of a small or large sample. If the sample is small the temperature of the probe will change its temperature because as the sample and the probe reach temperature equilibrium the temperature of the sample has to change. This obviously doesn't cause a significant error if the sample size is much larger than the temperature probe. The same applies to testing pH and the buffering capacity of the sample. colorpHast stripsEMD chemicals make a range of pH test strips that are a significant improvement over litmus paper or other economic pH test strips. The product is called colorpHast and has these advantages:
The type most suitable for brewing is the strips with a pH range of 4.0 - 7.0 (see Figure 7). Another important advantage is that they don't require calibration like a pH meter does. But their precision is limited to ~+/- 0.2 pH unit which is sufficient for testing the mash pH but some brewers like to have greater precision in their pH measurements. In comparative tests between the colorpHast strips and a pH meter a constant error of about 0.3 pH units was noticed when they are used in the 5.0 - 6.0 pH range; i.e. the strips read about 0.3 pH units lower than the pH that was determined with a pH meter. More info on the precision of the colorpHast strips can be found here: | |
pH meters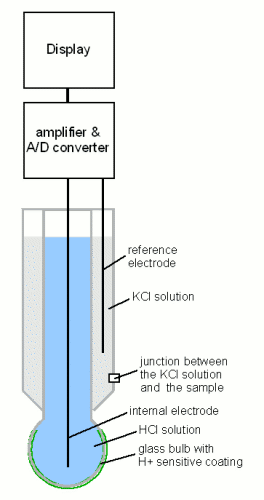 Figure 8 - schematic drawing of a pH meter A pH meter consists of 3 parts: a pH probe that converts the pH of the solution into an electrical potential, an amplifier and analog digital converter that converts that electrical potential into a pH reading that is then shown on the display. The heart of the pH meter is the glass bulb at the tip of the pH probe. It is coated on both sides with an ion selective hydrated gel which acts like a weak acid [ktf-split.hr], i.e. the pH of the surrounding medium determines how many of its molecules lost their protons (H+) (see figure 2). The pH inside the bulb, and with it the level of deprotonation, is fixed through a solution of hydrochloric acid (HCl). But the pH to which the outside layer is exposed is not fixed and it determines the level of deprotonation of that layer. If it is different from the inside layer an electrical potential is created which is picked up with the 2 electrodes. The stronger the pH difference the greater this electrical potential will be. The potential is then amplified and converted to a pH reading with the help of calibration settings. This pH value is then shown on the display. A completely different approach for taking pH readings is the use of an electronic pH meter. The heart of these meters is a glass electrode which converts the H+ concentration into a voltage that can be measured with a voltmeter. Since this voltage is proportional to the logarithm of the H+ concentration it is also proportional to the pH; in other words the pH of the sample is a linear function of the voltage that is measured at the probe. Every linear function is determined by 2 parameters: offset and slope. The slope can be calculated and is about 60 mV per pH unit [Wikipedia-3] but can change as the probe ages. A single point calibration uses a single solution of known pH. This solution is called a calibration buffer and the pH for common buffers are 4.00, 7.00 and 10.00. Since such a single point calibration can only determine the current offset of the probe a better calibration procedure uses 2 buffers. Most brewing related samples have a pH below 7 and calibrating the pH meter with 4.00 and 7.00 pH buffers is recommended. This calibration should be carried out regularly. Especially when the probe has not been used for a while. When the pH of a sample is measured it is also important to know the temperature of the sample. This is important for 2 reasons. The temperature of the sample affects its H+ concentration and therefore its pH. This is the result of changing H+ and OH- dissociation balances in the sample and is substrate specific. For wort it has been reported that the pH at mash temperatures (65 C/150 F) is about 0.35 pH units lower than at room temperature and at mash out temperatures (75 C/170 F) it is even 0.45 pH units lower [Briggs, 2004]. While my own experiments showed only a ~0.2 pH difference between a sample at mash temperature and a sample at room temperature (25 C/77 F). The temperature dependent change of pH is also important for the calibration of the pH meter since the buffer solutions have their nominal pH only at a specific temperature. This temperature is usually room temperature (25 C/77 F) and many buffer manufacturers supply tables with their buffers that show how their pH changes with temperature. I have made it a habit to always calibrate my pH meter with buffer solutions at room temperature (25 +/- 1 C). In addition to the pH of the sample the temperature also affects the response of the pH electrode. This effect is specific to the electrode and will not change from sample to sample that's why many pH meters are equipped with automatic temperature correction (ATC) where a separate temperature sensor, which in less expensive meters is oftentimes integrated into the probe, is used to measure the temperature of the sample and used to correct for the temperature error of the pH electrode. This means that the pH measured with an ATC pH meter still changes based on temperature but that change is only the result of temperature dependent H+ concentration in the sample. It is a true pH change in the sample. If the pH is also dependent on temperature what temperature should a sample be measured? The answer is that any temperature works as long as it is noted and reported with the pH measurement. To make comparing of pH measurements easier it is best to take them at a standard temperature; 25 C/77 F or 20 C/68 F are common. Some brewers use their pH meter to test the pH directly in the mash. But testing hot liquids reduces the lifetime of the probe which is why I don't recommend this practice. We will come back to this topic when we look at the target pH for mashing and boiling. |
|
|
The increased precision of a pH meter comes at a price. Not only are they more expensive, the probes need replacement after 1-2 years. Good care can extend the life of the probe. Here are some tips when working with pH meters:
|
|
|
A short buying guide for pH meters can be found here: pH Meter Buying Guide References
AcknowledgementsI'd like to thank the members of the Northern Brewer and HomeBrewTalk.com forums that helped with reviewing and proof reading the article. And special thanks to analytical chemistry professor J. Schneider who took time to review the article and give it his professional blessing.
|