An Overview of pH
|
ContentsWhat is pH ?pH is a measure of the acidity of a solution. It is a measure of the concentration of hydrogen ions (H+; proton). The higher the concentration of hydrogen ions in a solution the more acidc it is and the lower their concentration the more basic it is. In pure water (H2O) not all of the hydrogen (H) and oxygen (O) ions are bound in water molecules. A small number of these molecules are broken up (disassociated) into protons (H-) and hydroxide (OH- ions (see figure 1). The ion H+ concentration of freshly distilled water, which is water that didn't have a chance to pick up any minerals or gases corresponds to a pH of 7 which is the neutral pH. A acidic solution will have a pH below 7 and a basic solution has a pH greater than 7. Once distilled water stands in air it will pick up CO2 and the pH falls to about 6.5. (Technically free protons (H+) don't exist in water. They react with water molecules to form a hydronium ion (H3O+ but for the sake of simplicity in this article I will refer to them as protons as all that matters is their concentration and their electrical charge) The pH scale is not a linear measure of the proton concentration but a logarithmic one. This means that a change of 1 pH unit doesn't change the proton concentration by 1/7 of that of distilled water. Instead a solution with a pH of 6 has a 10 times higher proton concentration compared to distilled water. And a pH of 5 means it has a 100 times higher proton concentration. Likewise a solution with a pH of 8 has only 1/10 the proton concentration of distilled water. For each pH unit up the concentration is divided by 10 and for each pH unit down it is multiplied by 10. weak acids and bases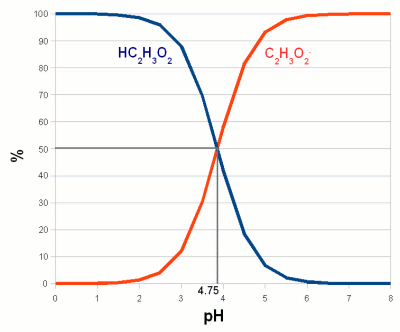 Figure 2 - The behavior of a weak acid at different pH. The relationship between the percentage of an acid (acetic acid (vinegar), C2H4O2) and its conjugate base (acetate, C2H3O2-). The pH at which the concentration of acid and its conjugate base are equal is called pKa and depends on the type of acid and the temperature. For acetic acid at 20C (68F) it is 4.75. Below that pH an increasing amount of acetate anions will absorb protons and become acetic acid. Above that pH an increasing amount of acetic acid will loose protons and become acetate anions. Or to express it another way, in order to decrease the pH protons have to be added to convert acetate anions into acetic acid When we later discuss the effects of pH we will come across one common theme: the disassociation of H+ and OH- ions from larger molecules. A substance that donates H+ to or accepts OH- ions from its environment is called an acid. It lowers the pH. A substance that accepts H+ or donates OH- is called a base and it raises the pH. When acids and bases are brought together they neutralize each other by forming a salt and water. When strong acids or bases are dissolved in water they readily dissolve and each molecule donates H+ or OH-. Depending on the concentration of the acid or base the result is a dramatic pH drop towards 0 or a pH rise towards 14. But most acids and bases we will deal with in brewing, especially when we talk about the effects of pH, are weak acids. A weak acid is an acid where only a portion of its molecules disassociate in water. The exact ratio depends on the pH of the solution, temperature and the type of acid. The same is true for weak bases. When an acid disassociates it leaves behind a negatively charged remainder, called conjugate base. Since the proportion of disassociated weak acid molecules depends on the pH, pH controls how many negatively charged conjugate bases are present. In case of weak bases the remainder (called conjugate acid) that is left after the disassociation of a hydroxyl ion (OH-) or the acceptance of a proton is positively charged. pH controls their concentration as well. Figure 2 illustrates the relationship between the ratio of acid and conjugate bases on the example of acetic acid (vinegar). Later we will see how the weak acids like tannins and complex molecules build from many different weak acids and weak bases (proteins for example) are affected by the pH of their environment though the pH dependent disassociation of H+ and OH-. pH buffersAnother basic pH related chemistry phenomenon are pH buffers and their buffer capacity. pH only tells us about the balance between protons (H+) and hydroxyl (OH-) in a solution. It doesn't tell us how strongly it is held in place. I.e. it may take the addition of only little amounts of an acid or a base to change the pH or it may take a lot to change it. What is keeping the pH in place are weak acids, bases and their salts. They all act act as pH buffers. 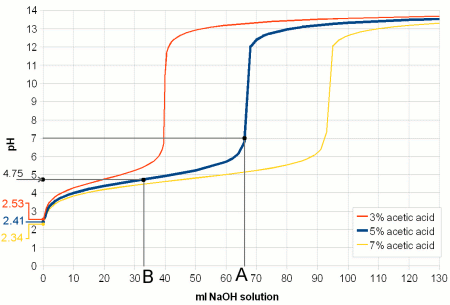 Figure 3 - Titration of vinegar. A strong base (sodium hydroxide, a.k.a. lye, NaOH) is added to 100ml of 3 different concentrations of acetic acid (vinegar). The pH of a pure acetic acid solution depends on its concentration and is shown on the left. As the base is added it converts more and more of the acetic acid to acetate. The ratio of acetic acetate and acetic acid determines the pH according to Figure 2. The solution acts as a buffer and the pH changes little with increasing additions of sodium hydroxid. At point B (in case of the 5% acetic acid solution) 50% of the acertic acid has been converted to acetate and the pKa of 4.75 is reached. At point A all of the acetic acid has been converted and the pH shoots up. The buffering capacity of the acetic acid/acetate buffer has been consumed by the sodium hydroxide and now the sodium hydroxide controls the pH. Since it is a strong base is quickly raises the pH towards 14. The process of adding controlled amounts of acid or bases to a sample is called titration and can be used to determine the buffer capacity of that sample. We saw in the last section that the ratio between conjugate base and acid molecules and their respective acid and base molecules depends on the pH and the type of acid/base itself. Lets look at a solution of acetic acid and acetate. At a pH of 4.75 half of the molecules are still acetic acid and the other half is its conjugate base acetate. When we now try to lower the pH by adding more H+ to the solution through the addition of a strong(er) acid the ratio of acetic acid and acetate molecules shifts towards acetic acid (Figure 2). This means that some of the H+ that was added will be consumed by creating more acetic acid molecules from the existing acetate molecules. This in turn reduces the number of protons that are available to lower the pH and as a result the pH doesn't fall as much as it would have if the lactic acid was not present or present at a lower concentration. The lactic acid and acetate mix acted as a buffer. The amount of stronger acid that needs to be added to lower the pH depends not only on the desired pH difference but also on the concentration of acetic acid and acetate. The higher their concentration the more acid it takes to lower the pH. This also works in the other direction when a strong base is added. In this case the rise in pH requires that more acetic acid is converted into acetate. This liberates protons which fight the pH rise. This is illustrated in Figure 3 In mashing and in beer there is not a single acid or base that acts as a buffer but a wide variety which have different pKa. The combined effect of all these buffer systems determines the pH of the wort or beer. And later when we talk about water we will come back to the major buffering system which are the various forms of carbonates: carbonic acid, bicarbonate and carbonate. Their concentration tell how much acid it takes to move the water pH into a favorable pH for mashing. Buffer solutions also provide a stable pH reference for the calibration of pH meters. These solutions have been mixed precisely from acids/bases and their salts to hold their pH steady even in the presence of small contamination with other acids or bases. isoelectric point (IEP)So far we have seen how pH controls the percentage of weak acids and weak bases that loose a proton or accept one respectively. This creates a charged molecule. Many complex molecules (amino acids and proteins for example) contain a number of groups that can loose or accept protons based on pH. The overall electrical charge of the molecule is the sum of all the charged groups. If there are acidic and basic groups on the molecule, i.e. groups that can become negatively charges (acidic) and groups that can become positively charged (basic) there will be a pH at which the sum of the electrical charges is zero and the molecule is electrical neutral. This pH is called the isoelectic point (IEP) and it depends in on the substance. In particular it depends on the pH characteristics of its acidic and basic groups. Figure 4 illustrates the IEP on Glutamic acid, one of the different amino acids that are the building block of peptides and proteins. 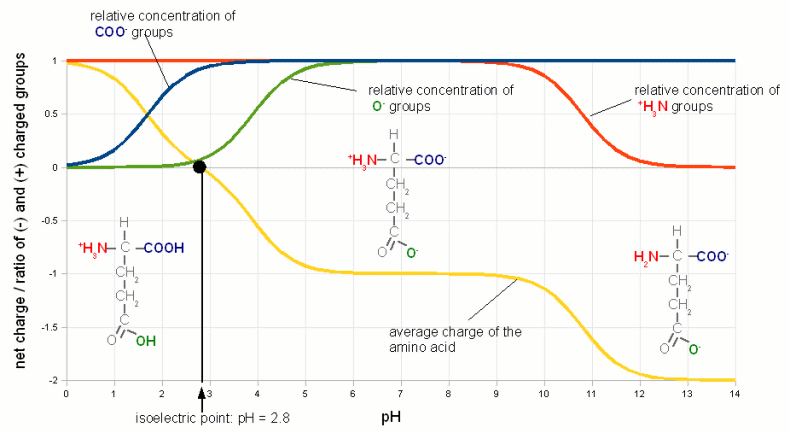 Figure 4 - The isoelectric point of Glutamic acid. Glutamic acid has 3 groups that can loose or accept proton and therefore change their electrical charge depending on the pH. The relative concentration of the charged state of these groups is shown with the gree, blue and red graphs. The yellow graph is the total charge of the amino acid. At pH 2.8, its isoelectric point, this charge is 0. (pKa data for Glutamic acid was taken from [Champe, 2007]) We will see later how the IEP and the pH dependence of electrical charges of various substances are of importance in brewing. At a pH below its IEP the net charge of a molecule is always positive because the higher concentration of H+ leads to more acceptance of H+ by the molecule's acidic and basic groups. The opposite is true at a pH above its IEP where the low H+ concentration causes the basic and acid groups to donate H+ to the environment which leaves the net charge on the molecule to be negative. Some readers may wonder how can the electrical charge of a molecule change while the solution it is in is still eclectically neutral. The answer is that the balance is provided by cations (Na+, Ca2+, etc.), anions (SO42-, HCO3-, etc) and other charged molecules which are necessary to hold the pH at its particular level. Measuring pHNow that we looked the chemical affects that are important for understanding pH lets have a look at how pH can be measured. For the home brewer 3 viable methods of testing pH exists: economy pH paper, colorpHast strips and pH meters. In general the cheaper the means of pH testing the less accurate the result is. pH indicator solutionspH indicator solutions are like weak acids and bases, they disassociate into a conjugate base or base depending on pH. But both the acid and the conjugate base (acid which lost one or more protons) have different colors. And because the relative concentration of acid and conjugate base changes with color (see Figure 2) the color of the indicator solution also changes with pH. A number of different indicator substances have been discovered each of which works best in its own pH range. A simple pH indicator can be made from red cabage juice and its color for different pH is shown in Figure 5. While this is great for illustrating pH indicators it has little practicality in brewing since the precision of pH readings is too low to be useful.
Lithmus paper and economy pH stripsWhen the aforementioned pH indicator solution is applied to filter paper and dried simple pH indicator strips can be made. These strips, sometimes called lithmus paper, are the cheapest means of testing the pH of a solution. To test the pH of a sample a strip is dipped into the sample. After removing it from the sample the color reaction is compared to a color scale that is supplied with the strips. The pH value shown for the color that matches the strips color reaction is the approximate pH of the sample. Since the indicators itself act as acids or bases they actually change the pH of the tested solution if it is not sufficiently buffered. It is important to think about the buffering capacity of the solution that is tested for pH with any of the means of testing pH. The less its buffering capacity the less accurate the pH measurement will be. Luckily most solutions that we test for pH in brewing are strongly buffered and we don't have to worry about this effect. The only one that is not strongly buffered is very soft or even distilled water. Here you can easily get false readings but the pH of the brewing water is of little importance anyway, especially when buffered only weakly, as we will see later. A good analogy to to testing the pH of strongly and weakly buffered solutions is measuring the temperature of a small or large sample. If the sample is small the temperature of the probe will change its temperature because as the sample and the probe reach a temperature equilibrium the temperature of the sample has to change. This obviously doesn't cause a significant error if the sample size is much larger than the temperature probe. The same applies to testing pH and the buffering capacity of the sample. colorpHast stripsEMD chemicals makes a range of pH test strips that are a significant improvement over litmus paper or other economic pH test strips. The product is called colorpHast and has these advantages:
Another important advantage is that they don't require calibration like a pH meter does. But their precision is limited to ~+/- 0.2 pH unit which is sufficient for testing the mash pH but some brewers like to have greater precision in their pH measurements. In comparative tests between the colorpHast strips and a pH meter I noticed a constant error of about 0.3 pH units. I.e. for most of their pH range the strips read about 0.3 pH units lower than the pH that was determined with a pH meter (More info can be found here colorpHastStrips vs. a pH meter and here micro mashing experiments pH metersA completely different approach for taking pH readings are electronic pH meters. The heart of these meters is a glass electrode which converts the H+ concentration into a voltage that can be measured with a volt meter. Since this voltage is proportional to the logarithms of the H+ concentration it is also proportional to the pH in other words the pH of the sample is a linear function of the voltage that is measured at the probe. Every liner function is determined by 2 parameters: offset and slope. The slope can be calculated and is about 60 mV per pH unit [7]. This slope may change as the probe ages. A single point calibration uses a single solution of known pH. This solution is called a calibration buffer and common buffer pH are 4.00, 7.00 and 10.00. Since such a single point calibration can only determine the current offset of the probe a better calibration procedure uses 2 buffers. Since most brewing related samples have a pH below 7 calibrating the pH meter with a 4.00 and a 7.00 buffer is recommended. This calibration should be carried out regularly. Especially when the probe has not been used for a while. When the pH of a sample is measured it is also important to known what the temperature of the sample is. This is important for 2 reasons. The temperature of the sample affects its H+ concentration and therefore its pH. This is the result of changing H+ and HO- disassociation balances in the sample and is substrate specific. In some cases the pH rises with the temperature or falls as the temperature rises or even follows a curve. For wort it has been reported that the pH at mash temperatures (65C/150F) is about 0.35 pH units lower than at room temp and at mash out temperatures (75C/170F) it is even 0.45 pH units lower [8]. The temperature dependent change of pH is also important for the calibration of the pH meter since the buffer solutions only have their nominal pH at a specific temperature. This temperature is usually room temperature (25C/77F) and many buffer manufacturers also supply tables with their buffers that show how their pH changes with temperature. I have made it a habit to always calibrate my pH meter with buffer solutions at room temperature (25C +/- 1). In addition to the pH of the sample the temperature also affects the response of the pH electrode. This effect is specific to the electrode and will not change from sample to sample that's why many pH meters are equipped with automatic temperature correction (ATC) where a separate temperature sensor, which is oftentimes integrated into the probe, is used to measure the temperature of the sample and and taken to correct for the temperature error of the pH electrode. This means that the pH measured with an ATC pH meter still changes based on temperature but that change is only the result of temperature dependent H+ concentration in the sample. If the pH is also dependent on temperature what temperature should a sample be measured at? The answer is that any temperature works as long as it is noted with the pH measurement. But to make comparing of pH measurements easier it is best to take them at the standard room temperature of 25C or 77F. Some brewers use their pH meter to test the pH directly in the mash but testing hot liquids reduces the lifetime of the probe which I don't recommend this practice. We will come back to this topic when we look at the target pH for mashing and boiling. The increased precision of a pH meter comes at a price. Not only are they more expensive, the probes need replacement after 1-2 years. Good care can extend the life of the probe. Here are some tips when working with pH meters: - always store in a pH meter storage solution and never in water even it it is distilled water. A pH meter storage solution contains salts that help the pH meter recover. - never let the probe dry out - thoroughly rinse the probe with distilled or deionized water, after removing from the storage solution, between measuring samples or calibration buffer and before returning to the storage solution. I flick with the wrist also removes the excess water from the probe. - measur cooled samples only - don't scratch or rub the glass bulb. It is coated with a sensitive layer that is integral to its function and it is rather fragile References
|

