Difference between revisions of "Building brewing water with dissolved chalk"
| Line 6: | Line 6: | ||
The acidic environment of a mash will eventually dissolve that chalk but not as complete as one would think. [http://braukaiser.com/documents/effect_of_water_and_grist_on_mash_pH.pdf Experiments] with dissolved and undissolved chalk have shown that the alkalinity potential of undissolved chalk is limited. In particular it was only able to raise the mash pH by up to 0.2 pH units regardless how much was added. Dissolved chalk, on the other hand, did not show these limitations. | The acidic environment of a mash will eventually dissolve that chalk but not as complete as one would think. [http://braukaiser.com/documents/effect_of_water_and_grist_on_mash_pH.pdf Experiments] with dissolved and undissolved chalk have shown that the alkalinity potential of undissolved chalk is limited. In particular it was only able to raise the mash pH by up to 0.2 pH units regardless how much was added. Dissolved chalk, on the other hand, did not show these limitations. | ||
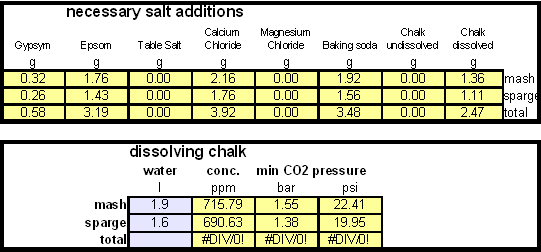
| − | While chalk can be dissolved in water using acids, it defeats the purpose since the use of acids counteracts the alkalinity that the chalk is supposed to give the water. Only when chalk is dissolved by using CO<sub>2</sub> is it able to raise the alkalinity of the water. The addition of CO<sub>2</sub> creates carbonic acids which is able to convert the chalks carbonate ion (CO<sub>3</sub><sup>2-</sup>) to a bicarbonate ion (HCO<sub>3</sub><sup> | + | While chalk can be dissolved in water using acids, it defeats the purpose since the use of acids counteracts the alkalinity that the chalk is supposed to give the water. Only when chalk is dissolved by using CO<sub>2</sub> is it able to raise the alkalinity of the water. The addition of CO<sub>2</sub> creates carbonic acids which is able to convert the chalks carbonate ion (CO<sub>3</sub><sup>2-</sup>) to a bicarbonate ion (HCO<sub>3</sub><sup>-</sup>). While doing that the carbonic acids itself is converted to bicarbonate. In the end one dissolved molecule of chalk (CaCO<sub>3</sub>) contributes 2 bicarbonate ions (HCO<sub>3</sub><sup>1-</sup>) and 1 calcium (Ca<sup>2+</sup>) ions. |
Revision as of 00:10, 10 December 2009
|
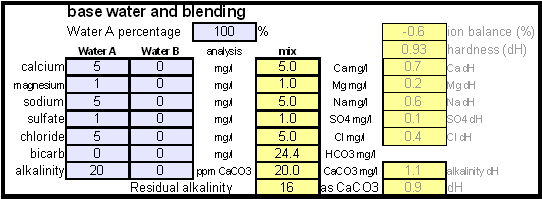
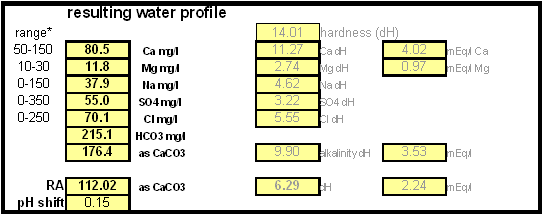
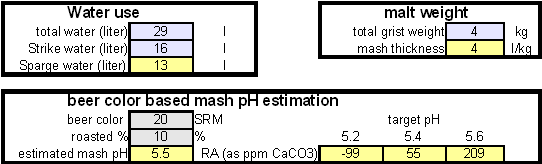
In order to be able to build brewing water with a profile that matches a particular city or to build any brewing water that has temporary hardness chalk (CaCO3) is needed. Chalk, however, is not very soluble in water and most of what needs to be added remains suspended or settles to the bottom. The acidic environment of a mash will eventually dissolve that chalk but not as complete as one would think. Experiments with dissolved and undissolved chalk have shown that the alkalinity potential of undissolved chalk is limited. In particular it was only able to raise the mash pH by up to 0.2 pH units regardless how much was added. Dissolved chalk, on the other hand, did not show these limitations. While chalk can be dissolved in water using acids, it defeats the purpose since the use of acids counteracts the alkalinity that the chalk is supposed to give the water. Only when chalk is dissolved by using CO2 is it able to raise the alkalinity of the water. The addition of CO2 creates carbonic acids which is able to convert the chalks carbonate ion (CO32-) to a bicarbonate ion (HCO3-). While doing that the carbonic acids itself is converted to bicarbonate. In the end one dissolved molecule of chalk (CaCO3) contributes 2 bicarbonate ions (HCO31-) and 1 calcium (Ca2+) ions.
|