Tonight I was finally able to do cell counts on an experiment that I wanted to do for a while now: “How does the access to air affect yeast growth in starters”. The experiment compared stirred starters capped with airlock, aluminum foil, no cover and injected air.
The setup was very simple. 8.9 Plato wort was prepared using DME and water (no hops) and inoculated with a 2 day old culture of WY2042 (Danish Lager). The innoculation rate was 10.8 B/l or 0.12 B/g (billion cells per gram of extract). The inoculated wort was split between four 500 ml Erlenmeyer flasks. Each flask received about 275 g wort.
All 4 flasks were placed onto identical stir plates set to identical speed in a temperature controlled incubator. The temperature during the experiment was 22 C.

Setup for the yeast growth over “access tro air” experiment
The air injection was done with sterile air and the end of the glass tube was positioned about 5 mm over the wort surface. This was done to avoid foaming and allowed air to be absorbed through the vortex.
All starters were allowed to grow for 2 days. After that time no more CO2 escape was noticeable in the airlock covered starter.
Results and Discussion
The following chart shows the amount of growth as billion new cells per initial gram of extract that grew in each of the starters. The error bars are based on a counting error of 10% that was applied to both the initial and the final cell count. Given the small number of initial cells the error of the final cell count is dominant in this case.
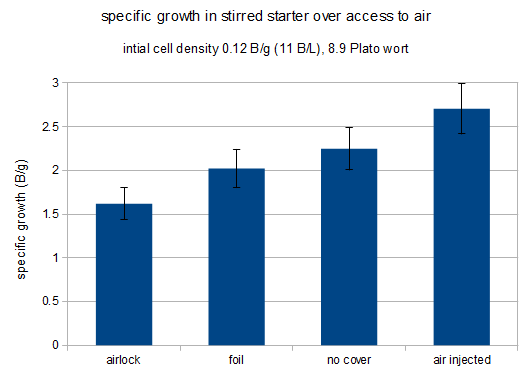
specific growth for each of the starters
It is apparent that increased access to air results in more yeast growth. In a previous experiment (not published) that compared an airlock against a foil coveted stirred starter the air lock covered starter showed a growth of 1.0 B/g for the air lock covered starter and 1.8 B/g for the foil covered starter. That result was more dramatic than the difference shown here.
The stream of air injected into one of the starters was so intense that it actually caused significant evaporation. It lead to a weight loss of 20% compared to the 2.2-3.2% for the other starters. Final cell counts considered the actual final volume.
The increased yeast growth could be caused by 2 different mechanisms.
- the more air that is available the more aerobic metabolism the yeast is able to perform. That means more energy for growth since aerobic metabolism is able to generate more ATP per mole of glucose than anaerobic metabolism. But in worts with high levels of sugars, as it is the case here, aerobic metabolism is limited by the Crabtree Effect.
- yeast growth in wort is limited by available oxygen for sterol production. That means that access to more O2 allows more cells to be grown.
This experiment is not able to shed light onto this and more experimentation is needed. Since yeast are able to absorb sterols from the growth medium the addition of olive oil to stirred and airlock covered starters could show if sterol synthesis is a limiter to growth in starters.
Conclusion
The better access a starter has to fresh air the more yeast can be grown. More experimentation is needed to better understand the limiters to yeast growth in starters.
While a completely uncovered flask is not practical for yeast propagation in a brewery it was included to show how how much can can be gained by not restricting the gas exchange. For practical yeast propagation a more sanitary alternative would be necessary.


Nice experiment and interesting results.
One question about yeast quality: Even though more yeast are grown with an uncovered vessel, what is the quality of that yeast? I’m not sure I’d be up for pitching a starter that sat open for a few days. Need to weight the risk of contamination vs the extra yeast gained.
Yes, contamination is a concern when there is no cover on the flask. The idea was to not worry about that aspect of practical yeast propagation and to examine how much can be gained by not restricting the gas exchange. For practical yeast propagation this means the loser the foil the better or injecting sterile air.
I’ll make a note about this in the blog post.
what about looking at the growth rate per/g as a factor for the health an speed of the yeast used in the 15 days fermentation expirement. i dont have much clue about this. trying to learn so excuse my ignorance 😛
Great experiment!
Few questions about the air injection.
1. Air was injected continuously over the 2 days?
2. Do you know what the flow rate was of the injection?
3. If evaporation was ~20%, and density was ~20% over the “no cover”, then was total cell count between the two about the same?
Yes, the air was injected over the 2 days but I don’t know about the flow rate. Out must have been max of the aquarium pump plus filter.
I did account for the change in volume when counting cells and the bars show total growth per extract. This is independent of volume.
Where do you think a “breathable” foam stopper would fall? My guess would be better than the foil, but not as good as wide open.
Jamie,
I was anticipating that question. I don’t have a good answer, though. I would have to buy one and give it a try.
My guess would be not quite as good as foil, but close. Foil is exactly the same thing as wide open (if someone tapes it tight around the flask neck, they’re doing it wrong) and it’s sole purpose is to prevent airborne microorganisms from falling into the medium. That’s all. It should be loosely wrapped around the flask mouth to allow for unhindered gas exchange while not allowing anything to fall into the flask.
Breatable foam is much the same, but I think still obstructs the free gas flow a little.
Interesting experiment.
You may have mentioned this in your response to the first question, but is using foil an okay was to make a starter from a sanitary aspect. Im fairly new to making starters and am just wondering what you typically use to cover yours.
Thanks.
Foil cover is what most brewers use on their starters.
G’day Kai, interesting experiment.
Any thoughts to compare injecting O2 versus air through the wort (both into wort and not above the surface)? Or has that already been pretty much done in the research community?
And look forward to any trials with olive oil.
Cheers,
Tom.
(MaltyHops @ brewadelaide.com)
Tom, I would not use O2 unless the rate can be controlled. O2 in excess is toxic to most organisms including yeast. Air is also much cheaper.
I usually sanitize my O2 stone and tube and snake it under the foil to hang it just above the liquid level. I give it a 10-20 second shot at about 1L per minute to start and then every 4 hours or so if I am around(while stirring). I haven’t measured the DO as I don’t have a meter, but I have seen huge cell growth with that tactic and holding propagation temps at 75F.
Kai: Did you mean to say, “1.the more air that is available the more anaerobic metabolism the yeast is able to perform.” I think you meant “aerobic metabolism,” although the Crabtree effect limits this (as you said).
I would be interested to see the same experiment performed without a stir plate. I wonder how much the continual stirring allows for more access to oxygen versus a stagnant wort.
I’m curious, using the foil method, if the opening size of the vessel has any impact on the specific growth. For example using a beaker vs an Erlenmeyer flask vs something with a form factor similar to a petri dish.
That might be worth an experiment.
Kai
VERY cool experiment.
If I’m not mistaken, this test shows growth of 15x for the airlock method and up to 25x for air injection method? If so, this seems mostly academic (which is cool, I love science and experimentation so we can learn best practices) or applicable to large batch brewing as even 15x growth would be enough for a 40-60 bil count yeast pack to grow to 600-900 bil which is enough for any 5 gallon batch and almost any 10 to even 20 gallon batch (depending on ABV).
Am I accurate in this or am I missing something major?