I noticed that the topic “At what temperature should mash pH be measured” comes up once in a while. Just recently I had an e-mail and an on-line discussion with a fellow home brewers on the same subject.
Fact is that the pH of a solution changes with temperature. It is caused by a change of the dissociation constants of the various acids/bases that are in the solution. Even water is considered an acid since it can donate hydrogen ions, although in most cases it is not dominating pH at all. The extent of the change depends on the substance. By the same mechanism even the pH optimum of enzymes may shift with temperature it is also dependent on the ionization state of the acids in the protein. I believe that the 0.35 correction factor for mash temp (65 C) vs. room temp (25 C) pH contains both the aspect that the actual pH in the mash is lower at 65 C compared to 25 C and that the pH optimum of the amylase enzymes shifts a bit from the value that can be observed by room temperature mashing.
But none of this matters since by convention pH values in brewing are reported as the pH of a room temperature sample. This arises from the laboratory practice of cooling pH samples before pH is tested. While pH meters can correct for temperature and their probes may be able to withstand higher sample temperatures, testing only cooled sample extends the life of the probe. This common practice also means that reported pH optima and pH ranges are for room temperature samples even though the actual reaction happens at higher temperatures. A.J. deLange mentioned to me the “by convention” aspect which is an important argument in this discussion. “By convention” means that we could do it differently but we settled on this particular method in order to communicate our observations and recommendations more clearly. Just as an example, another brewing measurement where we have a convention is the expressing the extract content in specific gravity. Rather than Plato, which measures the extract content by weight and which is something that doesn’t change with temperature, specific gravity does change with temperature and we assume that all those measurements are corrected for temperature such that they apply to a 68 F sample. The practice is and should be done for pH measurements. To be exact you’ll have to cool hot samples and warm cold (e.g. beer) samples.
It’s also helpful to take into account how we arrived at these pH optima/ranges. They are determined by conducting a series of mashes (at correct mash temp for that enzyme) with differing pH. The pH is tested in a room temp sample. The amount of product produced during these reactions (sugar, for example) is then plotted over this room temperature pH.
The same is true with boil pH recommendations where kettle boils at different pH values were done to determine how wort quality changes when the boil pH changes.
One problem is that hardly any author is explicit about this. I assume that most of them see it as a given that they talk about pH from room temperature samples. Briggs was the only one I found that made a distinction. This lack of explicitness, if this is a word, seems to cause a lot of confusion with home brewers.
As for the origin of this confusion, I believe that early home brewing literature and publications are to blame. John Palmer’s 1st edition of “How to Brew” states this:
“When you mash 100% base malt grist with distilled water, you will usually get a mash pH between 5.7-5.8. (Remember, the target is 5.1-5.5 pH.)”
In this sentence he mixes room temp and mash temp pH values. The 5.7-5.8 base malt pH is correct when seen as the pH of a room temperature mash sample while the 5.1-5.5 pH target is only correct when seen as a mash temp pH target with a conversion factor of 0.35. With the correction the room temp sample pH target range is 5.45 – 5.85, which is more correct.
The pH optima that John cites for various enzymes seem to be mash temp pH values. He doesn’t quote a source but the only source that I found which lists mash temp pH values is Briggs’s Brewing Practice and Science book. In this he also gives room temp pH numbers.
I came across this inconsistency when I started reading more technical brewing literature. The pH optima listed for the enzymes for the given optimal mash pH ranges just didn’t line up with what I heard from other brewers. It took me a while and doing my own pH vs. conversion experiments to get a clearer picture of this topic.
And to answer the question that is most interesting to brewers, I believe that the optimal mash pH range is 5.3-5.5 for light beers and 5.4-5.6 for darker beers when testing a room temperature sample of the mash. This pH range is a good compromise between optimal enzyme activity, good boil pH and good cast-out wort pH.
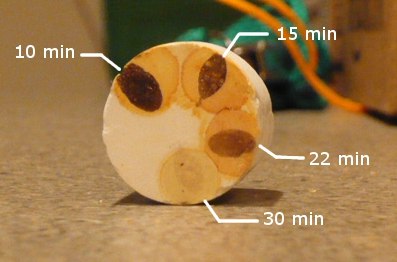 At the 30 min mark I considered the iodine test negative.
At the 30 min mark I considered the iodine test negative.